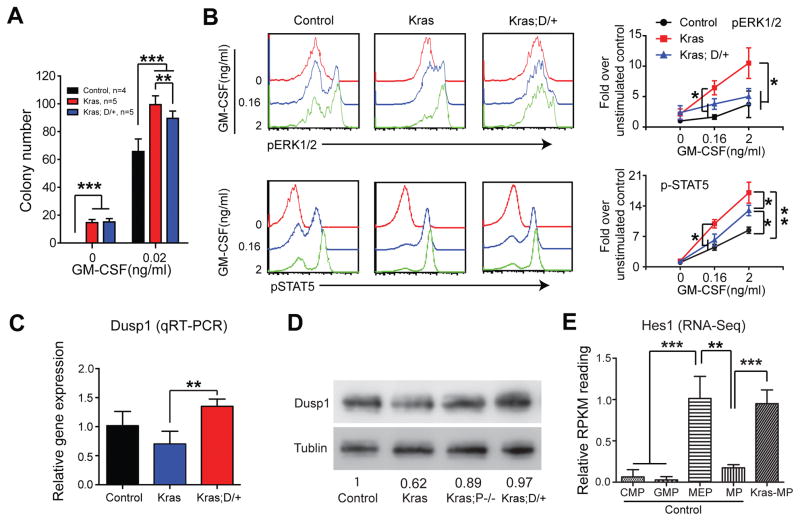
Figure 5. DNMAML expression leads to Dusp1 upregulation and downregulation of GM-CSF-stimulated ERK activation in Kras myeloid progenitors.
Lethally irradiated mice (CD45.1+) were transplanted with 2 ×106 splenocytes (CD45.2+) from KrasLSL G12D/+;Mx1-Cre (Kras) or KrasLSL G12D/+;Rosa26LSL DNMAML-GFP/+;Mx1-Cre (Kras; D/+) mice along with 2.5×105 competitor cells (CD45.1+). The control group was transplanted with 1×106 bone marrow cells (CD45.2+) along with 2.5×105 competitor cells (CD45.1+). Three weeks after transplantation, Cre expression was induced using pI-pC injections as described in Methods. Recipients transplanted with control, Kras or Kras; D/+ cells were sacrificed 4–5 weeks after pI-pC injections. Donor-derived cells are defined as CD45.2+ cells in control and Kras recipients or CD45.2+ GFP+ cells in Kras; D/+ recipients. (A) 5X104 donor-derived bone marrow cells from recipients were plated in duplicate in semi-solid medium with or without GM-CSF. (B) Donor-derived whole bone marrow cells were sorted using flow cytometry and serum- and cytokine-starved for 2 hours at 37°C. Cells were then stimulated with different concentrations of mGM-CSF for 10 minutes at 37°C. Levels of p-ERK1/2 and pSTAT5 were measured using phospho-flow cytometry. Lin−/low c-Kit+ cells, which are enriched for myeloid progenitors, were gated for analysis. (C, D) Dusp1 expression was quantified in donor-derived Lin− bone marrow cells using qRT-PCR (C) or Western blot (D). (E) Quantification of Hes1 expression in different populations of progenitor cells using RNA-Seq. Data are presented as mean ± SD. * P<0.05, ** P<0.01; *** P<0.001.