Abstract
Introduction:
The current “gold standard” to diagnose anal sphincter morphology and disruptions utilize low frequency (3-9 MHz) ultrasound (US) imaging techniques that provide a general outline of the sphincter muscles, but not their microstructural details. High frequency US transducers (7-15MHz) have been used to study the muscle architecture (direction of muscle fascicles) in the limb muscle.
Aims:
The goal of our study was to visualize the microstructural anatomy of the anal sphincters, specifically the external anal sphincter (EAS), using high frequency US imaging.
Methods:
Studies were conducted in asymptomatic female and male subjects and patients with fecal incontinence. US images were acquired using a low frequency US (3–9 MHz) and high frequency (7–15MHz) US transducer. The latter was placed intra-anally to image the anal canal at 12, 9, 3 and 6 o’clock positions.
Results:
The low frequency US images revealed the general outline of the anal sphincter muscles. On the other hand, high frequency imaging visualized muscle fascicles and connective tissue inside the external anal sphincter (EAS). In FI patients, there was loss of muscle fascicles and alteration in the echo-intensity pattern in the region of damaged EAS suggestive of muscle fibrosis.
Conclusion:
High frequency ultrasound imaging is a powerful tool to visualize the microstructural details of the EAS. Our studies show that damage to the EAS muscle results in the alteration of its myoarchitecture, i.e., loss of muscle fascicles and increase in the muscle connective tissue.
Abbreviated abstract:
High frequency ultrasound (7–15MHz) has been used to study the muscle myoarchitecture in the external anal sphincter. In patients with fecal incontinence, a loss of muscle fascicles and alteration in the echo-intensity pattern in this region has been observed and measured.
Introduction
Damage to the muscles of pelvic floor, especially the anal sphincter muscles, internal anal sphincter (IAS), external anal sphincter (EAS) and the puborectalis muscle (PRM) are extremely common during vaginal child birth. Using an endo-anal US probe, Sultan et al and others found damage to the IAS and EAS in 25–35% of women following first vaginal delivery; a significant number of these patients develop anal/fecal incontinence1, 2. Studies show high prevalence of IAS, EAS and PRM defects/damage in patients with the fecal incontinence3, 4. US imaging of the anal sphincter muscle is currently the “gold-standard” to identify damage to anal sphincter muscles. Since the original description of 2D endo anal US imaging technique, it is now possible to acquire the 3D anatomy of anal sphincters using mechanical transducers. More recently, transcutaneous (also called as trans-labial or trans-perineal) 3D-US imaging, instead of endo-anal approach, to image the anal sphincters has also been used by several investigators5–8. The advantages of transcutaneous approach are, 1) it is patient friendly (does not require insertion of probe into the anal canal), 2) there is no distension/deformity of the anal canal related to the US probe insertion into the anal canal (approximately 15mm in diameter), and 3) anal sphincter and pelvic floor muscles can be visualized in the 3D.
Most of the US transducers used in anal sphincter imaging, including transcutaneous 3D probe, are relatively low frequency (3–9 MHz). The images acquired by these transducers visualize the general outline of the muscles, and not the microstructural details inside the muscle. All muscles are made of muscle fascicles and connective tissue surrounded by a fascial sheath. The muscle fascicles themselves are made up of muscle fibers, which in turn are made up of actin and myosin filaments organized as sarcomeres9. High frequency US imaging with 9–15 MHz transducers has been used in the limb muscle to visualize the arrangement of muscle fascicles and changes in pennation angle of the fascicles during contraction10, 11. The feasibility of high frequency US probe to image the anal sphincter muscle in the humans was first described by Timor-Tritsch et al in 200512, 13. Our goal was to characterize the morphology of anal sphincter muscles in detail, especially the EAS using high frequency ultrasound imaging. We also assessed patients with fecal incontinence in whom damage to the EAS was visualized in the region of 11–2 o’ clock position (most common site of anal sphincter injury) to determine if the microstructural morphology of the damaged EAS is different from the normal EAS.
Methods
Studies were conducted in normal healthy females (n=8, mean age = 30 years), males (n=5, mean age = 31 years) and eight female patients with symptoms of fecal incontinence with evidence of damage to the anal sphincter muscle on the low frequency 3D transperineal US imaging. The healthy asymptomatic nulliparous females and male subjects reported no symptoms of FI, no trauma or surgery to the perineum/anorectum, and had normal bowel movements (>3 bowel movements per week). The 8 female patients (mean age 67 years, range 42–81, BMI = 27.5, parity = 2.8) who were being evaluated for symptoms of fecal incontinence were also part of this study. The protocol for these studies was approved by the University of California San Diego Human Research Protection Program under protocol #111030, and each subject signed an informed consent form.
Transperineal Low Frequency (3–9 MHz) 3D-Ultrasound Imaging:
The 3D-US images of the anal sphincter muscles were acquired using a low frequency (3–9 MHz) transducer by placing it on the perineum (transperineal, also referred to as translabial approach), (Philips EPIQ5 ultrasound machine, 128 tightly curved volume, field of view 156˚x 85˚, broadband technology) as a 3D US volumes. Images were stored as DICOM files and analyzed using the Philips Q Lab™ (Philips Ultrasound, USA) software. The US images were viewed for the normal anatomy and presence of damage to the anal sphincter muscles.
Transperineal and Intra anal High Frequency (7–15MHz) Ultrasound Imaging
The high frequency US transducer, 7–15MHz (from Philips, L15–7io, hockey stick transducer, 128 linear transducers, 23mm aperture length, 10 mm width, broadband technology, unique acoustic lens for imaging at transducer surface) was used for these studies. Two approaches were used, 1) in female subjects the HF probe was positioned with the long length of transducer oriented in the right to left direction in the labial fourchette which provides an axial image of the anal canal. The transducer was then rotated 90 degree, i.e., in the crano-caudal direction that provides mid sagittal plane image of the anal canal (Figure 1D). In the male subjects the transducer was placed on the perineum to acquire transcutaneous axial and sagittal image. The HF US images were also acquired with the transducer placed inside the anal canal (Figure 1A, 1B and 1C) with the surface of the transducer facing 12 o’clock (anterior), 9 o’clock (right), 3 o’clock (left) and 6 o’clock (posterior) positions. In each of these positions several 2D US images were acquired, while the subject were at rest. All images were stored and visualized as DICOM files in the Philips Q Lab™ software (Philips, The Netherlands).
Figure 1:
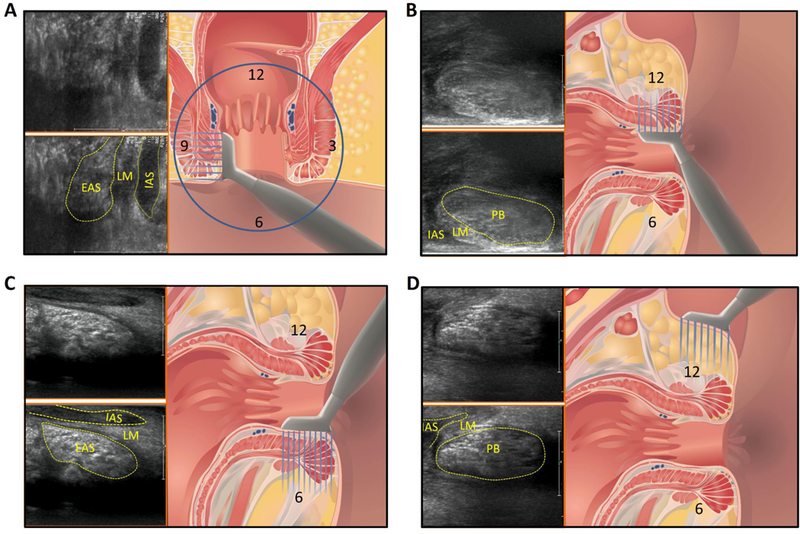
Schematic of the transducer placement and US images obtained at that location. IAS = internal anal sphincter, PB = perineal body, EAS = external anal sphincter, LM = longitudinal muscle. 12 ventral or anterior, 6 = dorsal or posterior, 9 = right and 3 = left
Data Analysis:
Images were analyzed in Image J (NIH, USA) and Matlab 2017b (Mathworks, Inc., Natick, Massachusetts, United States). The length, width and area of the perineal body and standard grayscale properties of the US image were assessed. Aside from the gray level intensity and standard deviation of the pixel values, four textural features were extracted from the gray level histograms and the gray level co-occurrence matrix (GLCM) of the HFUS perineal body data. These 4 features are, 1) Contrast: measures the local variation in GLCM; 2) Correlation: measures the joint probability occurrence of the specified pixel pairs. 3) Energy: provides the sum of the squared elements in the GLCM; and 4) Homogeneity which measures the closeness of the distribution of elements in the GLCM to the GLCM diagonal.
Statistical Analysis:
Data are reported as median and interquartile range. Wilcoxon rank sum test was used to measure the difference between the healthy and FI groups. p<0.05 was considered significant.
Results
Low vs High Frequency Axial and Sagittal Ultrasound Images of the Anal Canal
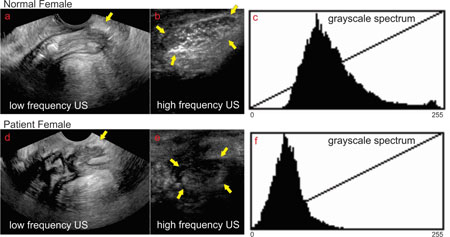
Axial views of the anal canal with low and high frequency US transducers in a normal female subject using transcutaneous approach are shown in the Figures 2A and2B respectively. Low frequency US image shows the entire anal canal with the surrounding muscles, IAS, EAS and perineal body (PB) (Figure 1A). In these images, general outline of the EAS muscles but not the arrangement of muscle fibers inside the EAS are seen. On the other hand, in the HF US image the structure close to the transducer, i.e., ventral end of the EAS (which is the same structure as the perineal body) but not the entire canal is seen in high resolution. Individual muscle fascicles traversing from right to left in the axial image can be seen (Figure 2B). A mid sagittal image of the anal canal with low and high frequency US image is shown in Figures 3A and3B respectively. The low frequency image shows the entire length of the anal canal with all surrounding muscles. Perineal body is seen as an oval shaped structure marked by the yellow arrow at the caudal end of anal canal (Figure 3A). The average length (cranio-caudal), width (dorso-ventral) and area of PB are 17.8 ± 2.8 mm, 6.5 ± 1.5 mm, and 8.8 ± 3.2 mm2 respectively. HF US image of the perineal body (Figure 3B) from the labial fourchette (position as shown in Figure 1D) in the sagittal plane shows an oval perineal body with white specks of different shapes (circular, oval and linear) inside the muscle. These white specks are the muscle fascicles running in many different directions.
Figure 2:
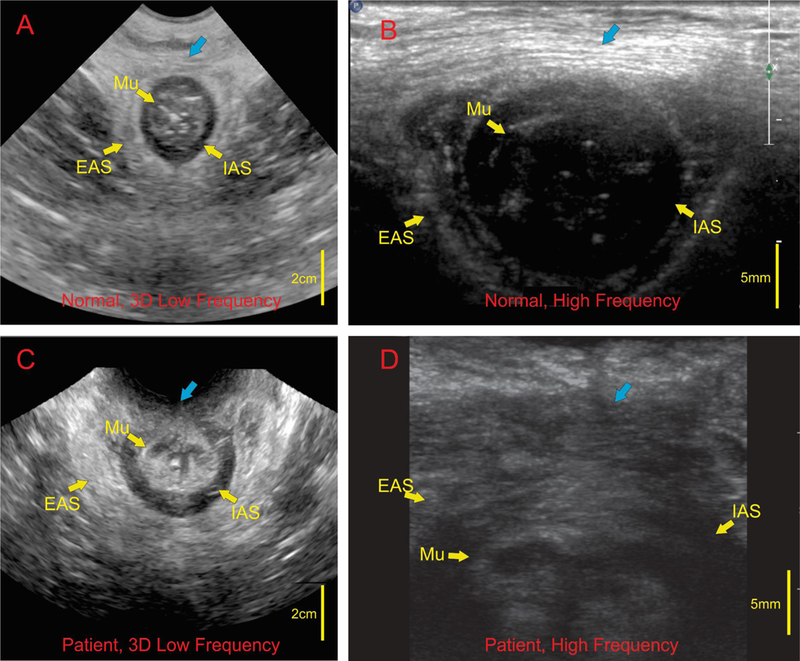
axial view of the anal canal using low and high frequency US imaging in a female subject: Images A and C represent low frequency images in a normal subject and patient with fecal incontinence respectively. Note different layers of the anal canal are seen, Mu (mucosa), IAS (internal anal sphincter) and EAS (external anal sphincter) in normal subject and disruption of the IAS and EAS in the patient. Blue arrows is the region of perineal body. High frequency images of the anal in a normal subject (B) show muscles fascicles in the perineal body traversing right to left. High frequency images in a patient (D) shows absence of regular muscle fascicles with irregular arrangement of muscle fascicles.
Figure 3:
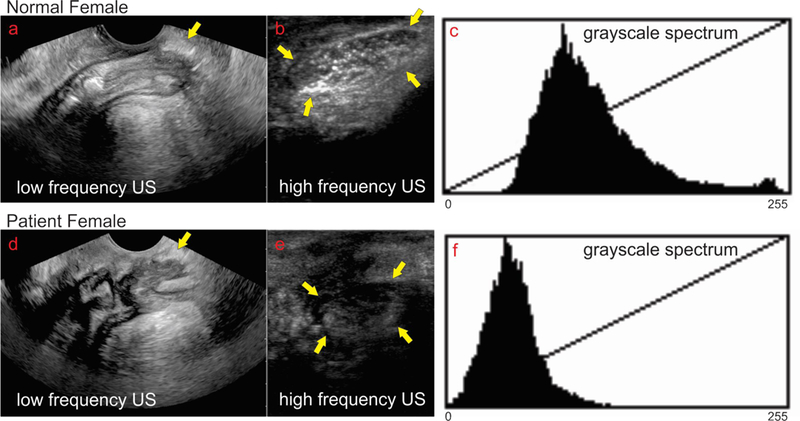
sagittal view low frequency vs high frequency in one normal subject and one patient with symptoms of fecal incontinence: low frequency sagittal image of the anal canal in a normal subject (a) and a patient with FI (d) shows the entire length of the anal canal. The oval shaped structure close to the yellow arrows is the region of the perineal body. High frequency image of the perineal body in normal subject (b) and patient (e). Note white speckles of varying shapes, circular and linear, are muscle fascicles inside the oval perineal body of normal subject (b). On the other hand, in the patient the US image of the perineal body appears darker with loss of white speckles, suggesting loss of microarchitecture. Figure c and f show the frequency distribution of the gray scale intensity of the structures insides the perineal body.
Intra-anal high frequency US images show muscle fascicles of the EAS in coronal planes at 3 and 9 o’ clock, and in mid sagittal plane at 12 and 6 o’ clock (Figures 1 and 4). At 12 o’ clock position, the PB appearance is similar to the one seen with transcutaneous imaging (Figure 2B), an oval shaped structure with bright specks of various shapes with intervening dark area that represents individual muscle fascicles and intervening connective tissue respectively. At 3, 6 and 9 o’ clock position one can see IAS, longitudinal muscle layer and EAS with the relatively well defined boundaries of each individual muscle. The EAS can be seen in entirety but only the caudal end of IAS and puborectalis muscles are seen (2.3 cm long transducer can’t reach more cranial structures). The PB and other structures of the anal canal can also be seen clearly in males, from the cutaneous as well as intra-anal approaches.
Figure 4:
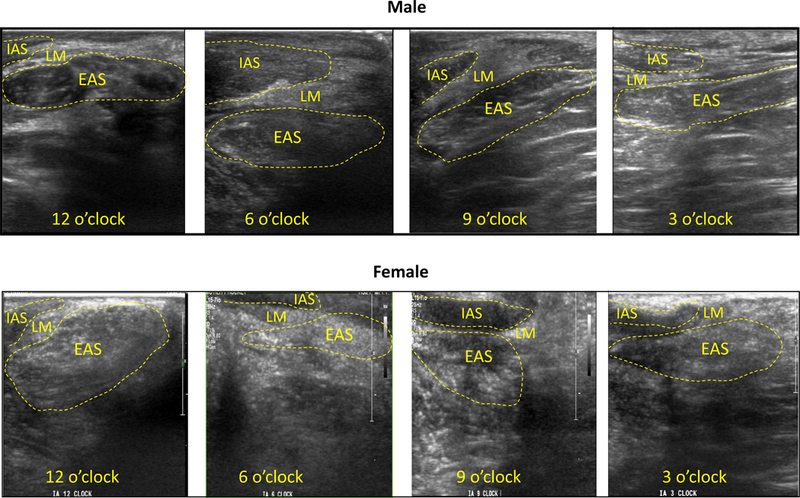
High frequency imaging of the anal canal from the intra-anal location. Top and bottom rows shows images from one normal male and female subject at intra-anal locations of 12, 6, 9 and 3 O’ clock positions respectively. Note different layers of the anal canal. The well demarcated perineal body (oval shaped structure) is seen clearly at 12 O clock position. At the other locations one can see the IAS, longitudinal muscle layer and EAS.
Figure 5 shows consistency of sagittal (A1, B1, C1 and D1), and axial (A2, B2, C2, D2) US images obtained by the HF US probe in 4 healthy female subjects. The PB was clearly identified as an oval shaped structure in males, it is somewhat longer in males as compared to females. The arrangement of muscle fascicles (white) inside the male PB (4A) is different as compared to females (most likely related to different direction/orientation of muscle fascicles with in the PB).
Figure 5:
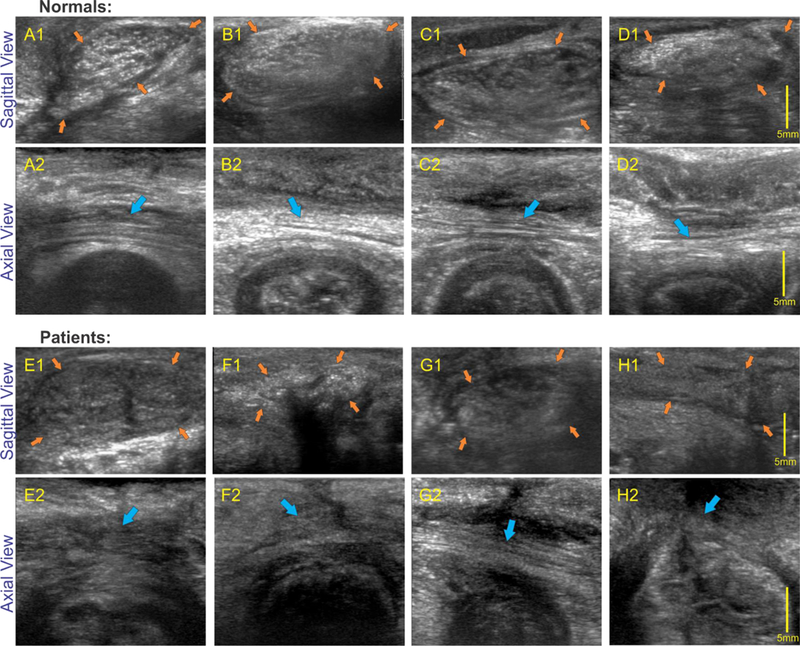
High frequency sagittal (perineal body) and axial images in normal and patients: upper 2 rows shows sagittal and axial images of the anal canal in 4 normal subjects and bottom two rows show sagittal images in 4 patients with fecal incontinence. Note that in all normal subjects oval shaped perineal body (orange arrows) is clearly visualized with white speckles of different shapes and sizes in axial images and linear white strands (muscle fascicles), marked by blue arrows, in the axial images of the perineal body. On the other hand, perineal body in patients is relatively dark and there is loss and disorganization of linear strands (muscle fascicles).
Figures 2C and 2D show low frequency and high frequency axial US images of the anal canal in a patient with FI. While the low frequency images show disruption of the EAS and IAS, the high frequency images show the absence of muscle fascicles in the PB. Low and high frequency sagittal US image of the anal canal and PB in a patient with FI are seen in figures 3D and3E. While low frequency images show oval shaped perineal body, the high frequency images shows PB to be relatively darker structure with loss of white specks seen so clearly in the normal subjects. The margins of perineal body are not well demarcated in the patient. Axial (5 E, FI, G1 and H1) and sagittal images (5E2, F2, G2, and H2) of perineal body in 4 patients are shows in Figure 5, again each of these patients shows disruption of the outline and loss of white speck inside the perineal body. The latter suggests disruption of the microarchitecture or loss of muscle fascicles.
Quantitative analysis of the high frequency image of the PB in normal and patients was performed using gray scale frequency spectrum analysis (Figure 3C and 3F) and texture analysis using gray level co-occurrence matrix analysis. The gray scale frequency spectrum analysis reveals that in the patient the frequency spectrum is shifted to the left and there is a narrower distribution of grayscale frequency spectrum. The latter suggests that the perineal body tissue is darker and more homogenous in the patient as compared to normal subject. The textural features from the of the normal subjects and FI patients are presented in Table 1. The grayscale, median, interquartile range,, smoothness, contrast correlation and homogeneity are significantly different in patients compared to normal subjects, which supports the visual US image appearance that in patient the PB is darker and more homogeneous, with fewer hyperechoic structures as compared to the normal subject.
Table 1:
Comparison of normal and patient HFUS perineal body features
| Perineal Body Features | Normals (n=8) | Patients (n=8) | |||
|---|---|---|---|---|---|
| Median | IQ | Median | IQ | p-value | |
| Intensity Mean | 87.1675 | 27.7935 | 57.7690 | 26.9100 | 0.0030* |
| Standard Deviation | 33.1774 | 10.9340 | 19.2515 | 2.7640 | 0.0003* |
| GLCM Contrast | 0.0348 | 0.0140 | 0.0170 | 0.0077 | 0.0002* |
| GLCM Correlation | 0.9695 | 0.0090 | 0.9507 | 0.0252 | 0.0104* |
| GLCM Energy | 0.8345 | 0.0417 | 0.8591 | 0.0973 | 0.1605 |
| GLCM Homogeneity | 0.9883 | 0.0024 | 0.9925 | 0.0036 | 0.0030* |
Discussion
In summary, our data shows the following; 1) low frequency US imaging provides a general outline of the anal canal and the surrounding muscles. On the other hand, HF US images reveal microstructural details, i.e., muscle fascicles inside the body of the muscle. Since perineal body, a part of the EAS and external anal sphincter are relatively superficial structures, they are easily accessible to the HF US imaging using the Philips hockey stick transducer. 2) In those patients with damaged EAS on the low frequency US imaging we found loss of muscle fascicles in the perineal body using high frequency US imaging. 3) The tissue in the PB of FI patients is more homogenous, which most likely represents fibrous tissue and not the functional muscle. 4) We found quantitative differences in the grayscale frequency spectrum analysis of the PB between controls and patients with damaged EAS, which may be of value in assessing the integrity of functional EAS muscle.
Ultrasound imaging is the current gold-standard to assess the integrity of anal sphincter muscle and has been in use since early 1990’s. The endo-anal ultrasound probes, approximately 15mm in diameter are generally used to image the anal sphincter muscle. Using mechanical low frequency US transducers it is possible to capture the anatomy of the anal canal in 3 dimensions (3D). More recently, several investigators have used the transcutaneous instead of endo-anal approach to image the anal sphincter and pelvic floor muscle in 3D. The US transducer used in the above systems is a relative low frequency (3–9MHz) transducer. The advantage of low frequency US imaging is that it images structure located far from the transducer, up to 5–8 cm distance away the transducer, but at the cost of resolution. The advantage of high frequency US transducer is that it visualizes structures close to the transducer at high resolution. Timor-Tritsch et al imaged the anal canal in female subjects from the labial fourchett, normal and patients, using high frequency probe that we used. The focus of our study was to visualize the microstructural details of the anal sphincter, especially the EAS which is amenable to HF US imaging because of its superficial location. In addition to imaging from the labial fourchette, we obtained images from the inside of the anal canal (which has never been reported), all around the circumference of the anal canal and identified all important layers of the anal canal. The structure that we could visualize in entirety was the perineal body and EAS because of the limited length of the transducer (2.3cm) that could not be advanced deep in the anal canal to visualize the IAS and PRM in entirety. In majority of patients the damage to the anal sphincter muscles occur between 11 o’ clock to 2 o’ clock position which is the location of the perineal body. What exactly is the PB? The classic text describes PB as a midline structure and the site of insertion of several muscles; EAS, superficial and deep transverse perinea and bulbospongiosus14. However, our work proves that the PB is the site of crossing of EAS muscle fascicles from right and left sides, which then continue as transverse perinea and bulbospongiosus muscles to be attached to the pubic rami of the opposite sides. The EAS is not composed of circular muscle fibers; rather it is shaped like a “purse string” with crossing of the two sides of EAS in the PB15. Therefore, we believe that PB is an extremely important part of the EAS muscle; it is also the most frequent site of damage in women due to childbirth related injuries and surgical episiotomy. HF US imaging clearly show a well-defined PB with muscle fascicle of different shapes and size inside it. The latter is because of different directions of muscle fascicles; if all the fascicles were traversing in a circular direction one would see the same shape of muscle fascicles in a midline sagittal image, which is not the case.
Damage to a muscle in general leads to the loss of the muscle fibers which is replaced by collagen and fibrous tissue16, 17. The latter has finer and fibrillary structure as compared to the relatively larger size muscle fascicles. We found that a surgical incision on the EAS muscle in rabbit leads to replacement of the muscle tissue by fibrous tissue which persists for 3 months with impairment of muscle function18. In FI patients, with an identifiable damage to the anal sphincter muscles on low frequency US imaging, we found that the definition of muscle fascicles in the perineal body was lost. The region appeared darker and more homogeneous. Analysis of US images using grayscale intensity analysis revealed that the tissue is darker in patients as compared to normal subjects. Furthermore, the frequency spectrum of echogenicity is narrower in patients as compared to normal subjects. The latter suggests homogeneity of tissue in the patients. The fibrotic tissue in general is thought to be less echo-lucent (white) than the normal tissue19. However, in the case of EAS the fascicles themselves have high echogenicity and in comparison the fibrous tissue is darker than the normal muscle. Loss of muscle fascicles in the damaged area of the muscle is therefore an expected finding. What does the homogeneity of the tissue implies? We suspect that the dark appearing homogeneous tissue is actually the fibrous tissue, which we is more homogenous than the muscle. However, confirmation of above will require muscle biopsy which is not practical and can be regarded as a limitation of our study.
High frequency US imaging is fairly simple and one can get adequate images of the EAS from the transcutaneous as well as intra-anal route. Since the probe is only 1 cm in width, insertion into the anal canal is quite easy and well tolerated by the subjects. The hockey stick probe is only 2. 3 cm in length and therefore it does not reach more cranial muscles of the anal canal, i.e., IAS and PRM in entirety. The strength of high frequency imaging is that it allows one to study the microstructural details that are not revealed by the low frequency imaging. It is possible that in some patients with a normal outline of the EAS muscle the defect may be loss of muscle fascicles and excessive fibrous tissue inside the body of the muscle. Such a muscle may appear intact on the low frequency imaging but will have abnormal muscle function as measured by anal manometry.
There are several limitations of our study; 1) we studied only small number of normal subjects and patients and therefore it is more of a feasibility study and only provides proof of the concept that myostructural details of the EAS, especially perineal body which can be assessed using high frequency US imaging, 2) we did not study the relationship between structure and function. We can’t recommend use of high frequency US imaging to assess the anal sphincter anatomy routinely in clinical practice based on our study. However, our study provides rationale and methodology which may be useful for future studies.
Acknowledgments
Financial Support: This work was supported by a NIH Grant DK
Footnotes
COI: None of the authors have any conflict of interest.
References
- 1.Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med 1993;329:1905–11. [DOI] [PubMed] [Google Scholar]
- 2.Sultan AH, Kamm MA, Nicholls RJ, et al. Prospective study of the extent of internal anal sphincter division during lateral sphincterotomy. Dis Colon Rectum 1994;37:1031–3. [DOI] [PubMed] [Google Scholar]
- 3.YS K, Weinstein M, Raizada V, et al. Anatomical disruption and length tension dysfunction of anal sphincter complex muscles in patients with fecal incontinence. Dis Colon Rectum 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharucha AE, Fletcher JG, Harper CM, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic faecal incontinence. Gut 2005;54:546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstein MM, Pretorius DH, Jung SA, et al. Transperineal three-dimensional ultrasound imaging for detection of anatomic defects in the anal sphincter complex muscles. Clin Gastroenterol Hepatol 2009;7:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim YS, Weinstein M, Raizada V, et al. Anatomical disruption and length-tension dysfunction of anal sphincter complex muscles in women with fecal incontinence. Dis Colon Rectum 2013;56:1282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valsky DV, Messing B, Petkova R, et al. Postpartum evaluation of the anal sphincter by transperineal three-dimensional ultrasound in primiparous women after vaginal delivery and following surgical repair of third-degree tears by the overlapping technique. Ultrasound Obstet Gynecol 2007;29:195–204. [DOI] [PubMed] [Google Scholar]
- 8.Guzman Rojas RA, Shek KL, Langer SM, et al. Prevalence of anal sphincter injury in primiparous women. Ultrasound Obstet Gynecol 2013;42:461–6. [DOI] [PubMed] [Google Scholar]
- 9.Lieber RL, Ward SR. Skeletal muscle design to meet functional demands. Philos Trans R Soc Lond B Biol Sci 2011;366:1466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawakami Y, Ichinose Y, Fukunaga T. Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol (1985) 1998;85:398–404. [DOI] [PubMed] [Google Scholar]
- 11.Ichinose Y, Kanehisa H, Ito M, et al. Relationship between muscle fiber pennation and force generation capability in Olympic athletes. Int J Sports Med 1998;19:541–6. [DOI] [PubMed] [Google Scholar]
- 12.Timor-Tritsch IE, Monteagudo A, Porges RF, et al. The use of a 15–7-MHz ‘small parts’ linear transducer to evaluate the anal sphincter in female patients. Ultrasound Obstet Gynecol 2005;25:206–9. [DOI] [PubMed] [Google Scholar]
- 13.Timor-Tritsch IE, Monteagudo A, Smilen SW, et al. Simple ultrasound evaluation of the anal sphincter in female patients using a transvaginal transducer. Ultrasound Obstet Gynecol 2005;25:177–83. [DOI] [PubMed] [Google Scholar]
- 14.Raizada V, Mittal RK. Pelvic floor anatomy and applied physiology. Gastroenterol Clin North Am 2008;37:493–509, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittal RK, Bhargava V, Sheean G, et al. Purse-string morphology of external anal sphincter revealed by novel imaging techniques. Am J Physiol Gastrointest Liver Physiol 2014;306:G505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieber RL, Ward SR. Cellular mechanisms of tissue fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am J Physiol Cell Physiol 2013;305:C241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol 2013;304:C216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajasekaran MR, Sinha S, Seo Y, et al. Myoarchitectural and functional alterations in rabbit external anal sphincter muscle following experimental surgical trauma. Am J Physiol Gastrointest Liver Physiol 2014;307:G445–51. [DOI] [PubMed] [Google Scholar]
- 19.Pillen S, Tak RO, Zwarts MJ, et al. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol 2009;35:443–6. [DOI] [PubMed] [Google Scholar]


