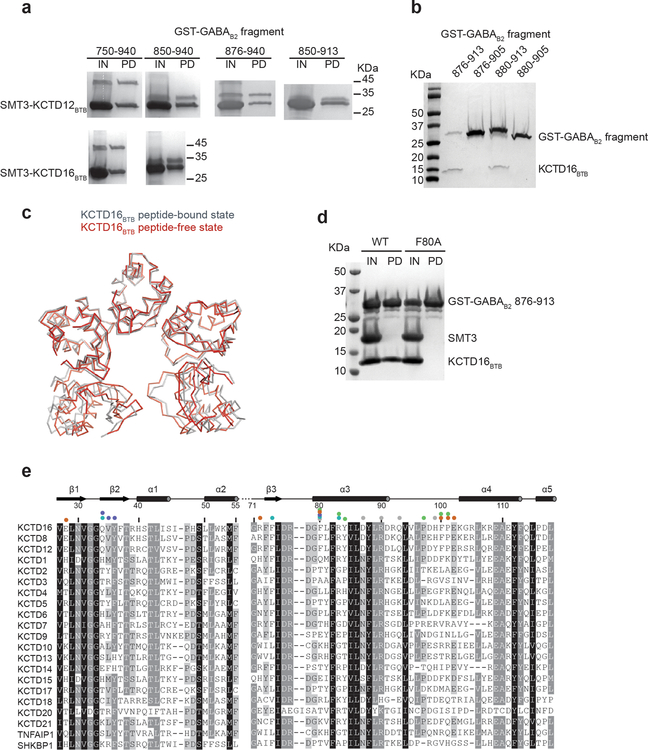
Extended Data Figure 1 |. Mapping the GABAB2 binding region of BTB domain.
a, His6-SMT3-tagged KCTD16BTB or KCTD12BTB and His6-GST-tagged GABAB2 peptides were co-expressed in E. coli and purified with nickel affinity chromatography. Purified protein were loaded as input for pull-down with glutathione sepharose beads. Glutathione input (IN) and pull-down (PD) fractions were analyzed by SDS-PAGE and Coomassie blue staining. b, His6-GST-tagged GABAB2 and untagged KCTD16BTB were co-expressed in E. coli. Clarified lysate was pulled down with glutathione Sepharose beads and eluate was analyzed by SDS-PAGE and Coomassie blue staining. c, Structural superposition of the KCTD16BTB structure in GABAB2 peptide-free (grey) and bound state (red; PDB ID 5A15). d, His6-SMT3-tagged KCTD16BTB wild-type (WT) or F80A mutant and His6-GST-tagged GABAB2 peptides were co-expressed and purified with nickel affinity. The eluate was treated with ULP1 to cleave SMT3 tag (IN) prior to glutathione Sepharose beads pull-down. e, Sequence alignment of the BTB domain of KCTD family members from Homo sapiens. Residues with 98%, 80% and 60% similarity are shown in black, grey, and light grey, respectively.