Abstract
Background
The relationship between mitral valve prolapse (MVP) and sudden cardiac death (SCD) remains controversial. In this systematic review, we evaluate the relationship between isolated MVP and SCD to better define a potential high‐risk subtype. In addition, we determine whether premortem parameters could predict SCD in patients with MVP and the incidence of SCD in MVP.
Methods and Results
Electronic searches were conducted in PubMed and Embase for all English literature articles published between 1960 and 2018 regarding MVP and SCD or cardiac arrest. We also identified articles investigating predictors of ventricular arrhythmias or SCD and cohort studies reporting SCD outcomes in MVP. From 2180 citations, there were 79 articles describing 161 cases of MVP with SCD or cardiac arrest. The median age was 30 years and 69% of cases were female. Cardiac arrest occurred during situations of stress in 47% and was caused by ventricular fibrillation in 81%. Premature ventricular complexes on Holter monitoring (92%) were common. Most cases had bileaflet involvement (70%) with redundancy (99%) and nonsevere mitral regurgitation (83%). From 22 articles describing predictors for ventricular arrhythmias or SCD in MVP, leaflet redundancy was the only independent predictor of SCD. The incidence of SCD with MVP was estimated at 217 events per 100 000 person‐years.
Conclusions
Isolated MVP and SCD predominantly affects young females with redundant bileaflet prolapse, with cardiac arrest usually occurring as a result of ventricular arrhythmias. To better understand the complex relationship between MVP and SCD, standardized reporting of clinical, electrophysiological, and cardiac imaging parameters with longitudinal follow‐up is required.
Keywords: mitral valve, sudden cardiac death, ventricular fibrillation, ventricular tachycardia
Subject Categories: Valvular Heart Disease, Sudden Cardiac Death
Clinical Perspective
What Is New?
Reported cases of isolated mitral valve prolapse and sudden cardiac death indicate that young females with bileaflet redundant leaflets are predominantly affected.
Clinical predictors of sudden cardiac death in isolated mitral valve prolapse are lacking.
The estimated incidence of sudden cardiac death in mitral valve prolapse is 217 events per 100 000 person‐years from previous studies.
What Are the Clinical Implications?
Further work is needed to understand the complex relationship between mitral valve prolapse and sudden cardiac death.
Standardized reporting of clinical, electrophysiological, echocardiographic, and other cardiac imaging variables with documentation of long‐term outcomes is required.
Mitral valve prolapse (MVP) is characterized by the atrial displacement of the mitral valve (MV) leaflet(s) during ventricular systole. The estimated prevalence of MVP is 2.4%, with approximately equal sex distribution.1
Although most MVP cases are thought to be benign, reported complications include mitral regurgitation (MR) requiring MV surgery, infective endocarditis, stroke, and sudden cardiac death (SCD).2 The association between MVP and SCD (a potential high‐risk MVP subtype) has been reported but the underlying mechanisms remain poorly understood. It is postulated that SCD in individuals with MVP is caused by ventricular arrhythmias (VAs),3, 4 although this association remains controversial.1, 2, 5 The initial description of MVP involved cardiac auscultation, cineangiography, and histopathological examination.6 This led to an abundance of literature describing MVP at autopsy,7, 8, 9, 10, 11 provoking discussions about a causal relationship between MVP and SCD.
The application of M‐mode and 2‐dimensional echocardiography for the diagnosis of MVP posed challenges as the identification of MVP shifted from the long axis view,12, 13 to either a long axis or apical 4‐chamber view,14 and then back to the long axis view as the gold standard for diagnosing MVP.15 These changes resulted in a significant rise and fall in the prevalence of MVP,1, 16 with implications for the estimated incidence of SCD.
We aimed to comprehensively evaluate all reported cases of MVP and SCD in the current literature to better characterize the potential high‐risk MVP subtype and to determine whether clinical and diagnostic parameters can predict which patients with MVP were at a higher risk of experiencing SCD. Furthermore, based on published studies, we provide an estimated incidence of SCD in MVP.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure as source data for this systematic review are available from web‐based medical libraries.
Case Identification and Search Strategy
We conducted a literature search for cases of MVP with SCD or cardiac arrest in PubMed and Embase on January 1, 2018, using Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.17 PubMed search terms were “mitral valve prolapse” AND “cardiac arrest” OR “mitral valve prolapse” AND “sudden cardiac death” OR “mitral valve prolapse” AND “sudden death” OR “mitral valve prolapse” AND “arrhythmia.” Embase search terms were “mitral valve prolapse” AND “heart ventricular fibrillation” OR “mitral valve prolapse” AND “heart arrest” OR “mitral valve prolapse” AND “sudden death” OR “mitral valve prolapse” AND “sudden cardiac death” OR “mitral valve prolapse” AND “heart ventricular tachycardia” OR “mitral valve prolapse” AND “heart arrhythmia” OR “mitral valve prolapse” AND “heart ventricular arrhythmia.”
Titles and abstracts were screened for relevance by 2 reviewers (H.H. and F.J.H.) and bibliographies of all included publications were screened to identify additional references. Screening of the above search result was also conducted to identify articles, which investigated whether patients with MVP had certain clinical, electrophysiological, or imaging predictors that were associated with VAs or SCD. Finally, prospective studies of patients with MVP, which reported SCD outcomes, were included to estimate the incidence of SCD in MVP. Details of the search algorithm are shown in Figure 1.
Figure 1.
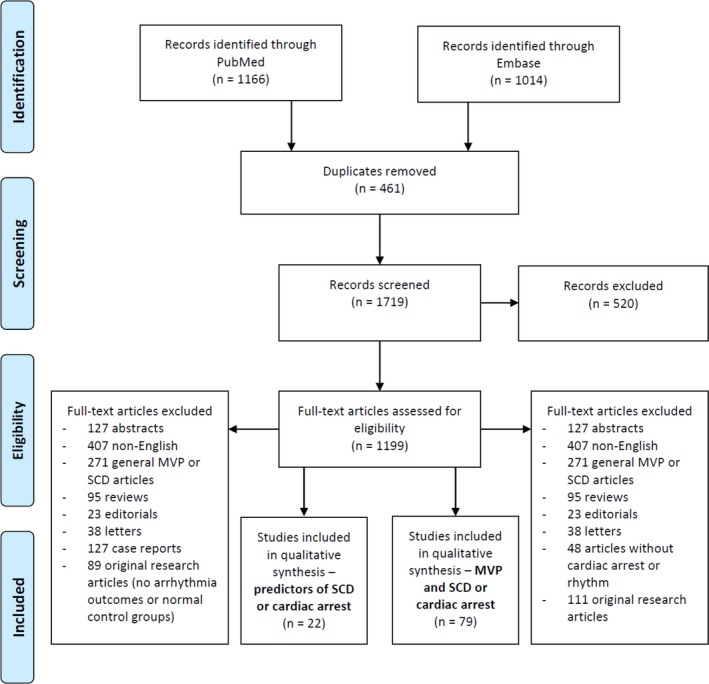
Search algorithm. MVP indicates mitral valve prolapse; SCD, sudden cardiac death.
Included articles were any cases of MVP with SCD or MVP with cardiac arrest and documented rhythm reported in English. Cases of MVP and SCD were separated into isolated MVP (iMVP) and nonisolated MVP (non‐iMVP) depending on whether there was another potential cause of death or cardiac arrest. Reports from case series were included if individual patient age and sex could be determined. Cases were excluded if they described VAs that did not result in cardiac arrest or survived cardiac arrest without a documented rhythm. Reports were also excluded if they were published only in abstract form.
Regarding predictors of SCD or VAs, we excluded articles that used healthy patients (as opposed to those with high‐ versus low‐risk MVP) as controls. We also excluded articles with nonsignificant findings or outcomes that were not related to VAs or SCD.
Regarding the incidence of SCD in MVP, we used prospective studies that included a mean patient age older than 18 years, at least 100 patients, and minimum follow‐up duration of 24 months.
Statistical Analysis
Continuous data are presented as either medians with interquartile ranges (IQRs) or means with SDs as indicated. Categorical data are presented as absolute numbers and percentages.
Results
In total, 161 cases of MVP with either SCD or cardiac arrest were identified from 79 studies, with 123 cases of iMVP and 38 cases of non‐iMVP. A further 22 studies investigated predictors of VAs or SCD. Comprehensive details of all included studies are presented in Tables S1 and S2. There were 3 studies that provided long‐term follow‐up data regarding SCD in MVP.18, 19, 20
Clinical Characteristics in iMVP and SCD
Clinical characteristics of the cases are summarized in Table 1. The age‐sex distribution of the index event of cardiac arrest or death is illustrated in Figure 2.
Table 1.
Baseline Characteristics in Cases of MVP and SCD or Cardiac Arrest
| Baseline Characteristics | All Cases (N=161) | iMVP (n=123) | Non‐iMVP (n=38) |
|---|---|---|---|
| Age, y | |||
| Range | 6–79 | 6–79 | 8–76 |
| Mean±SD | 37±16 | 36±16 | 40±17 |
| Median (IQR) | 32 (25–51) | 30 (25–47) | 36 (26–56) |
| Female sex | 109 (68) | 85 (69) | 24 (63) |
| SCD | 100 (62) | 75 (61) | 25 (66) |
| Circumstances of death or cardiac arrest | n=98 | n=74 | n=24 |
| Sleeping | 6 (6) | 5 (7) | 1 (4) |
| Normal daily activitya | 45 (46) | 34 (46) | 11 (46) |
| Exertion or soon afterb | 22 (22) | 17 (23) | 5 (21) |
| Emotional stress | 6 (6) | 4 (5) | 2 (8) |
| Driving | 4 (4) | 4 (5) | 0 |
| Anesthesia relatedc | 6 (6) | 5 (7) | 1 (4) |
| Pregnancy relatedd | 4 (4) | 3 (4) | 1 (4) |
| Witnessed in hospital | 5 (5) | 2 (3) | 3 (13) |
| Prior symptomse | n=71 | n=48 | n=23 |
| Dizziness | 14 (20) | 11 (23) | 3 (13) |
| Syncope | 25 (35) | 14 (29) | 11 (48) |
| Dyspnea | 9 (13) | 5 (10) | 4 (17) |
| Chest pain | 20 (28) | 15 (31) | 5 (22) |
| Palpitations | 39 (55) | 28 (58) | 11 (48) |
| Fatigue | 6 (8) | 4 (8) | 2 (9) |
| None | 12 (17) | 10 (21) | 2 (9) |
| Previous cardiac arrest | n=20 | n=14 | n=6 |
| Yesf | 8 (40) | 3 (21) | 5 (83) |
| No | 12 (60) | 11 (79) | 1 (21) |
| Medication use | n=57 | n=32 | n=25 |
| Digoxin | 7 (13) | 1 (3) | 6 (24) |
| β‐Blockerg | 16 (28) | 7 (22) | 9 (36) |
| Class 1h | 10 (18) | 0 | 10 (40) |
| Amiodarone | 1 (2) | 0 | 1 (4) |
| Other medicationsi | 15 (26) | 9 (28) | 6 (24) |
| Nil | 17 (30) | 16 (50) | 1 (4) |
| Family history of SCD | n=28 | n=22 | n=6 |
| Yes | 4 (14) | 3 (14) | 1 (17) |
| No | 24 (86) | 19 (86) | 5 (83) |
Values are expressed as number (percentage) unless otherwise indicated. iMVP indicates isolated mitral valve prolapse; MVP, mitral valve prolapse; IQR, interquartile range; SCD, sudden cardiac death.
Includes death at home, work (nonphysical), or during commute.
One case was after sexual intercourse.
Four cases during induction, 1 case during anesthesia reversal, and 1 case during peripheral arterial puncture.
Two cases were during pregnancy, 1 case during epidural injection, 1 case (classified as nonisolated mitral valve prolapse [non‐iMVP]) was 2 days postpartum with likely tachycardia‐mediated cardiomyopathy caused by permanent junctional reciprocating tachycardia.
Multiple symptoms in some cases.
Three cases with documented ventricular fibrillation.
Two patients taking sotalol (classified as non‐iMVP).
Includes propafenone, procainamide, mexilitine, quinidine, disopyramide, and flecainide.
Includes amoxicillin, diuretics, antiepileptics, primidone, methyldopa, perindopril, trastuzumab, inhaled glucocorticosteroids, danazol, domperidone, and various psychotropic agents in 3 cases.
Figure 2.
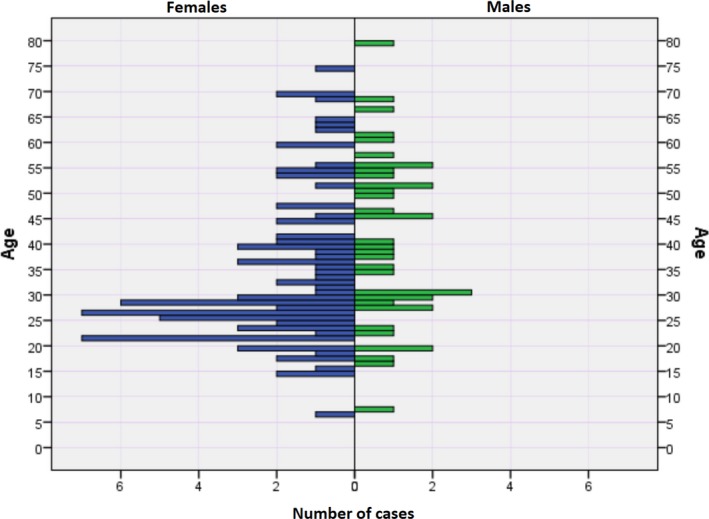
Age at time of death or cardiac arrest in mitral valve prolapse according to sex.
For patients with iMVP, the median age was 30 years (range 6 to 79 years), female sex accounted for 69% of cases, and 61% were SCD cases. The median age for female cases was 28 (IQR, 24–41) years and the median age for male cases was 39 (IQR, 28–53) years. Two cases occurred in individuals younger than 10 (ages 6 and 7), and a further 6 cases in individuals between 10 and 18 years. Activity at the time of cardiac arrest included routine daily activities (46%), exertion related (23%), emotional stress (5%), sleeping (7%), driving (5%), and pregnancy related (4%). Seven cases had cardiac arrest while in the hospital, with 5 occurring in the setting of general anesthesia.
Preceding symptoms included palpitations (58%), syncope (29%), chest pain (31%), dizziness (23%), and fatigue (8%). Only 21% of patients were reported to be asymptomatic before the index event. Three cases had a history of cardiac arrest, although none of these cases overlapped with those who had prior syncope.
Prior medication use was reported in 32 cases, of which 8 (25%) involved patients taking either a β‐blocker or digoxin at the time of cardiac arrest or SCD and 50% who were not taking any medications. One patient was taking multiple psychotropic medications,21 while another case described MVP and SCD in a patient with markedly elevated concentrations of caffeine (from an energy supplement).22
A positive family history for SCD was reported in 14% of cases. One case described a possible familial cluster of malignant MVP involving a 14‐year‐old female with SCD and iMVP, 3 first‐degree relatives with SCD (mother aged 36, sister aged 11, and brother aged 12 years who had thickening of his MV) and 3 of 7 remaining siblings with MVP.8
Electrophysiological Findings in iMVP and SCD
Electrophysiological findings for cases of MVP and SCD or cardiac arrest are shown in Table 2.
Table 2.
Electrical Findings in Cases of MVP and SCD or Cardiac Arrest
| Electrical Findings | All Cases | iMVP | Non‐iMVP |
|---|---|---|---|
| Baseline ECG changesa | n=81 | n=59 | n=22 |
| Inferior TWIb | 15 (19) | 14 (24) | 1 (5) |
| Other ST‐T changesc | 16 (20) | 11 (19) | 5 (23) |
| PVCsd | 40 (49) | 30 (51) | 10 (45) |
| Normal | 23 (28) | 19 (32) | 4 (18) |
| Atrial fibrillation | 9 (11) | 5 (8) | 4 (18) |
| Left ventricular hypertrophy | 5 (6) | 2 (3) | 3 (14) |
| Othere | 9 (11) | 5 (8) | 4 (18) |
| Holter findings | n=36 | n=24 | n=12 |
| No PVCs | 4 (11) | 2 (8) | 2 (17) |
| PVCs and couplets only | 20 (56) | 15 (63) | 5 (42) |
| Nonsustained VT | 10 (28) | 7 (29) | 3 (25) |
| TDP/VF | 2 (6) | 0 | 2 (17) |
| Cardiac arrest rhythm | n=72 | n=53 | n=19 |
| VF | 58 (81) | 43 (81) | 15 (79) |
| VT | 9 (13) | 6 (11) | 3 (16) |
| TDP | 3 (4) | 2 (4) | 1 (5) |
| Asystole | 2 (3) | 2 (4) | 0 |
| PVS findings | n=26 | n=22 | n=4 |
| Normal | 13 (50) | 12 (55) | 1 (25) |
| Nonsustained VT | 6 (23) | 5 (23) | 1 (25) |
| Sustained VT | 2 (8) | 1 (5) | 1 (25) |
| VF | 5 (19) | 4 (18) | 1 (25) |
| Site of origin of PVCs or VT | n=10 | n=6 | n=4 |
| Left ventricle | 3 (30) | 2 (33) | 1 (25) |
| Right ventricle | 5 (50) | 4 (67) | 1 (25) |
| Both | 2 (20) | 0 | 2 (50) |
Values are expressed as number (percentage). MVP indicates mitral valve prolapse; PVS, programmed ventricular stimulation; SCD, sudden cardiac death; TDP, torsades de pointes; VF, ventricular fibrillation; VT, ventricular tachycardia.
Multiple changes in some cases.
All leads (11 cases), lead III (1 case), leads II and III (2 cases), and leads III and aVF (1 case).
T‐wave inversion (TWI) in lateral leads (7 cases), TWI in V1–V3 (1 case), diffuse changes (1 case), and not specified (7 cases).
Includes multiple premature ventricular complexes (PVCs) (1), multifocal PVCs (6), bigeminy (3), and couplets (1).
Includes premature atrial complexes, bundle branch blocks, and accessory pathway (isolated mitral valve prolapse [iMVP] cases); Brugada pattern, prolonged QT, left axis deviation, and poor R‐wave progression (nonisolated mitral valve prolapse [non‐iMVP] cases).
On baseline ECG, premature ventricular complexes (PVCs) were frequently reported (51%), while T‐wave inversion in the inferior leads (24%) and other T‐wave changes (19%) were also common. Seven cases described combined inferior and lateral T‐wave changes. Normal baseline ECG findings were described in 32% of cases.
Among patients who underwent Holter monitoring, PVCs and couplets were the most common finding (63%), followed by nonsustained VT (29%). No abnormalities were recorded in 8%.
The site of origin of VT or PVCs was available (either reported or interpreted based on published ECG) in 6 cases. Both left and right bundle branch morphologies (in V1) were present with regard to VT or PVC origin. Four cases (all VT) published 12‐lead ECGs allowing for interpretation of possible VT origin (Figure 3).23, 24, 25, 26 Cardiac arrest rhythm was reported in 53 cases and was caused by ventricular fibrillation (VF) (81%), VT (11%), torsades de pointes (4%), and asystole (4%). Six cases documented the initiation of malignant VAs with 5 cases showing PVC‐triggered polymorphic VT or VF (Figure 4).24, 27, 28, 29, 30, 31 In total, there were 10 cases of autopsy‐confirmed MVP (6 with iMVP and 4 with non‐iMVP) with documented cardiac rhythm at the time of death, and they all had VF.10, 22, 29, 32, 33, 34, 35, 36, 37, 38
Figure 3.
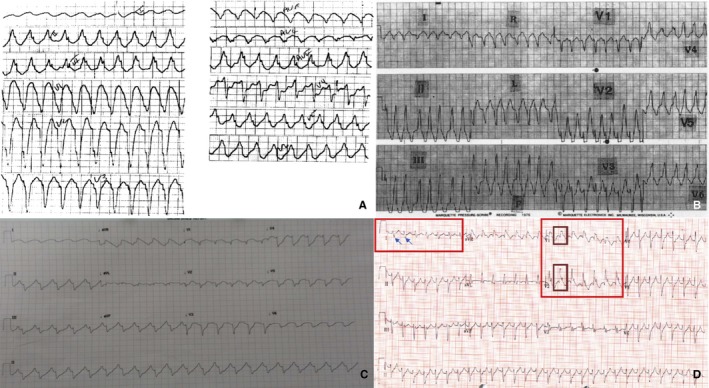
Twelve‐lead ECGs of ventricular tachycardia. Left and right bundle morphology interpretation based on V1 appearance. A, Left bundle morphology, inferior axis (isolated mitral valve prolapse [iMVP], reproduced with permission from Elsevier).23 B, Left bundle morphology, inferior axis (nonisolated iMVP [non‐iMVP], patient taking procainamide, reproduced with permission from Elsevier).24 C, Left bundle morphology, superior axis (iMVP, reproduced with permission from BMJ Publishing Group Ltd.).25 D, Right bundle morphology, superior axis (iMVP, reproduced with permission from Elsevier).26
Figure 4.
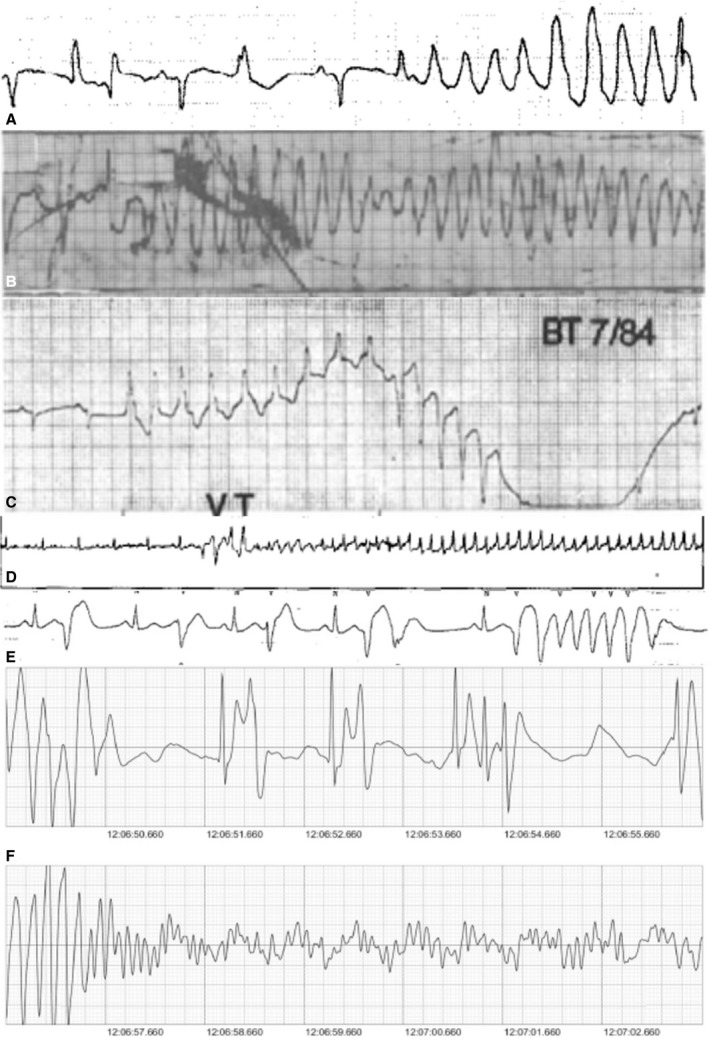
Documented onset of ventricular arrhythmias. A, Late diastolic premature ventricular complex (PVC)–triggered polymorphic ventricular tachycardia (VT; nonisolated mitral valve prolapse [non‐iMVP], patient taking quinidine, reproduced with permission from Elsevier)27 B, Possible PVC‐triggered polymorphic VT (isolated mitral valve prolapse [iMVP], reproduced with permission from Elsevier)28 C, Monomorphic VT with pace termination (non‐iMVP, patient taking procainamide, reproduced with permission from Elsevier)24 D, Late diastolic couplets triggering polymorphic then fast VT (non‐iMVP, patient had arrhythmogenic right ventricular cardiomyopathy, reproduced with permission from Elsevier)29 E, Late diastolic PVC–triggered polymorphic VT with varying PVC morphologies in rhythm strip (iMVP, reproduced with permission from Elsevier)30 F, (bottom 2 strips), PVC–triggered recurrent VF (iMVP, reproduced with permission from Elsevier).31
Programmed ventricular stimulation was reported for 22 cases using various induction protocols. The findings included sustained VT (5%), nonsustained VT (23%), VF (18%), and no induction of VAs (55%).
Cardiac Imaging Findings in iMVP and SCD
Cardiac imaging findings for cases of MVP and SCD or cardiac arrest are shown in Table 3.
Table 3.
Imaging Findings in Cases of MVP and SCD or Cardiac Arrest
| Imaging Findings | All Cases | iMVP | Non‐iMVP |
|---|---|---|---|
| Leaflet involvementa | n=83 | n=57 | n=26 |
| Bileaflet | 57 (69) | 40 (70) | 17 (65) |
| Posterior leaflet | 23 (28) | 15 (26) | 8 (30) |
| Anterior leaflet | 3 (4) | 2 (4) | 1 (4) |
| MR severity | n=38 | n=23 | n=15 |
| Nil/trivial | 9 (24) | 6 (26) | 3 (20) |
| Mild | 12 (32) | 9 (39) | 3 (20) |
| Moderate | 8 (21) | 4 (17) | 4 (27) |
| Severe | 9 (24) | 4 (17) | 5 (33) |
Values are expressed as number (percentage). iMVP indicates isolated mitral valve prolapse; non‐MVP, nonisolated mitral valve prolapse; MVP, mitral valve prolapse; MR, mitral regurgitation; SCD, sudden cardiac death.
Determination based on either noninvasive imaging reports and/or autopsy reports.
Leaflet involvement was most commonly bileaflet (70%), then posterior leaflet (26%) and anterior leaflet (4%). Severe MR was present in 17% of cases. Six cases reported MV surgery (3 repair and 3 replacement), with 3 cases describing improvement in VAs (follow‐up duration ranged from 2 to 3 years), 2 cases describing recurrent VT requiring treatment even after surgery, and 1 case with unreported arrhythmia outcomes.
Two cases reported cardiac magnetic resonance imaging findings, with 1 case reporting anteroseptal and posterior left ventricular wall fibrosis, while the other did not demonstrate late‐gadolinium enhancement.
Cardiac Structural Findings in iMVP and SCD
Cardiac structural findings are summarized in Table 4.
Table 4.
Cardiac Structural Findings Based on Autopsy Reports, Surgical Reports, or Cardiac Investigations
| Cardiac Structural Findings | All Cases | iMVP | Non‐iMVP |
|---|---|---|---|
| Mitral valve changes | n=88 | n=73 | n=15 |
| Redundant leaflet(s)a | 87 (99) | 72 (99) | 15 (100) |
| Annulus circumference, mmb | n=19 | n=15 | n=4 |
| Range | 96–160 | 100–160 | 96–135 |
| Median, IQR | 125 (100–136) | 126 (113–138) | 106 (97–120) |
| Anterior leaflet length, mm | n=15 | n=13 | n=2 |
| Range | 20–35 | 20–35 | 20–28 |
| Median, IQR | 30 (25–30) | 30 (25–30) | |
| Posterior leaflet length, mm | n=16 | n=13 | n=3 |
| Range | 15–30 | 15–30 | 15–30 |
| Median, IQR | 25 (20–30) | 25 (20–30) | 28 |
| Chordal changes | n=56 | n=45 | n=11 |
| Normal | 3 (5) | 2 (4) | 1 (9) |
| Abnormalc | 37 (66) | 28 (62) | 9 (82) |
| Ruptured | 16 (29) | 15 (33) | 1 (9) |
| Left ventricle histology | n=40 | n=30 | n=10 |
| Normald | 20 (50) | 18 (60) | 2 (20) |
| Abnormale | 20 (50) | 12 (40) | 8 (80) |
| Other cardiac abnormalities | n=50 | n=27 | n=23 |
| Left ventricular hypertrophy or cardiomegaly | 14 (28) | 0 | 14 (61) |
| Right ventricular fibrosisf | 6 (12) | 5 (19) | 1 (4) |
| Coronary artery diseaseg | 6 (12) | 0 | 6 (26) |
| Otherh | 6 (12) | 5 (19) | 1 (4) |
| Nil | 18 (36) | 17 (63) | 1 (4) |
IQR indicates interquartile range.
Includes descriptive terms myxomatous, ballooned, thickened, nodose, hooding, floppy, voluminous, opaque, and edematous.
Three additional cases reported a dilated annulus without measurement.
Descriptions included elongated, thickened, and/or fused.
Fifteen normal samples were from 1 series (all samples in that series were normal).11
Heterogeneous group of descriptors including fibrosis affecting the interventricular septum (3), interstitial fibrosis (5), extensive papillary muscle fibrosis (1), slight papillary muscle fibrosis (2), subendocardial fibrosis affecting the papillary muscles (2), presence of myxomatous material within the papillary muscles (1), multifocal necrosis (3), high‐grade left ventricular hypertrophy changes (1), and degenerated elastic fibers (1).
One case with arrhythmogenic right ventricular cardiomyopathy (nonisolated mitral valve prolapse [non‐iMVP]).
Includes left main coronary disease (1), anomalous right coronary artery (2), coronary vasospasm (1), prior inferior infarct (1), and significant diffuse coronary disease in the setting of pseudoxanthoma elasticum (1).
Includes tricuspid valve prolapse (3) and previous endocarditis (2) (isolated mitral valve prolapse cases) and significant conduction system fibrosis (1) (non‐iMVP case).
Autopsy confirmation of MVP was documented in 73 of the 75 SCD cases. In total, 72 of 73 (99%) cases that commented on the MV described redundant leaflets. Median MV annulus circumference was 126 mm based on 15 cases, while another 2 cases reported a dilated annulus. Median anterior and posterior MV lengths were 30 mm and 25 mm, respectively. Leaflet thickness was not reported in cases of iMVP and SCD. Chordae were described in 45 cases and included generalized abnormalities (62%), rupture (33%), and normal appearance (4%).
Histological abnormalities in the left ventricle were described in 12 of 30 cases (40%), with 3 cases describing fibrosis involving the papillary muscles. From 27 cases that described other cardiac structural findings, 17 cases (63%) had no other abnormal findings, 5 cases (19%) had right ventricular fibrosis, 3 cases (11%) had tricuspid valve prolapse, and 2 cases (7%) had evidence of prior endocarditis.
Nonisolated MVP Cases
For cases of non‐iMVP, there were 11 cases with a probable other cause of death or cardiac arrest including anomalous right coronary artery (2), significant left main coronary disease (1), diffuse coronary disease in the setting of pseudoxanthoma elasticum (1), coronary vasospasm (1), previous inferior infarct (1), arrhythmogenic right ventricular cardiomyopathy (1), Brugada syndrome (1), hypertrophic cardiomyopathy (1), dilated cardiomyopathy (1), and postpartum cardiomyopathy (1). There were a further 27 cases with another possible cause of death or cardiac arrest including nonspecific left ventricular hypertrophy or cardiomegaly (12), conduction system fibrosis (2), possible side effect from antiarrhythmic medications (13), and prolonged QTc (3) or a combination of the above. These cases are identified in Table S1.
Predictors of VAs and SCD
We identified 22 articles that reported a heterogeneous group of clinical, electrical, and imaging predictors for MVP and its association with various clinical outcomes. A summary of all studies is presented in Table 5, 3, 4, 18, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 and a full list is presented in Table S2.
Table 5.
Predictors of VAs or SCD
| Author | Year | Study population | Predictor/association | Outcome/Endpoint |
|---|---|---|---|---|
| Clinical | ||||
| Gaffney39 | 1979 | MVP |
Higher heart rate Lower cardiac index |
Clinical severity (combination of symptoms and VAs) |
| Puddu40 | 1983 | MVP | Plasma catecholamine level | QTc |
| Sniezek41 | 1992 | MVP | Adrenaline excretion | Complex VAs (Lown grade ≥3) |
| Zuppiroli42 | 1994 | MVP | Female | Complex VAs (Lown grade ≥3)a |
| Babuty43 | 1994 | MVP | Age (older) | Complex VAs (Lown grade ≥3) |
| Naksuk44 | 2016 | MV surgery | Age (younger) | PVC reduction post‐surgery in BiMVP |
| Fulton45 | 2017 | MVP | Female | PVCs from PM |
| Electrical | ||||
| Campbell46 | 1976 | MVP | Inferolateral T‐wave changes | VT (>100bpm for ≥3 beats) or VF |
| Babuty43 | 1994 | MVP | Late potentials | VT (≥3 beats) |
| Bobkowski47 | 2002 | MVP | Late potentials | VAs (Lown grade ≥1) and VT (>120bpm for ≥4 beats) |
| Akcay48 | 2010 | MVP | QTc dispersion | VT (>120bpm for ≥3 beats)a |
| Imaging | ||||
| Shah49 | 1982 | MVP | MR | Complex VAs (Lown grade ≥3) |
| Nishimura18 | 1985 | MVP | Redundant leaflets | Sudden deatha |
| Kligfield5 | 1985 | MVP | MR | VAs (>1% PVC frequency or exercise induced PVCs/VT or Lown grade ≥4 complex VAs) |
| Sanfilippo50 | 1989 | MVP |
Anterior leaflet thickness MR |
VAs (≥10 PVCs/hr or VT at ≥100bpm for ≥3 beats) |
| Zuppiroli42 | 1994 | MVP | Anterior leaflet thickness | Complex VAs (Lown grade ≥3)a |
| Babuty43 | 1994 | MVP | MR | Complex VAs (Lown grade ≥3) |
| Zouridakis51 | 2001 | MVP |
MVP degree Anterior leaflet thickness |
QT dispersiona |
| Turker52 | 2010 | MVP | Moderate‐severe MR | VAs (Lown grade ≥1)a |
| Carmo53 | 2010 | MVP | Mitral annular disjunction | Non‐sustained VT (NS) |
| Han54 | 2010 | MVP | LGE in PM | Complex VAs (Lown grade ≥4) |
| Akcay48 | 2010 | MVP | Anterior leaflet length | VT (>120 bpm for ≥3 beats)a |
| Sriram3 | 2013 | OHCA | BiMVP | Appropriate ICD therapies at follow‐up |
| Basso4 | 2015 | MVP | LGE | Complex VAs (Lown grade ≥4b or VF) |
| Nordhues55 | 2016 | MVP | BiMVP | All‐cause mortality |
| Bui56 | 2017 | MVP | Myocardial T1 time | Complex VAs (Lown grade ≥3) |
| Fulton45 | 2017 | MVP |
BiMVP LGE in PM |
PVCs from PM |
BiMVP indicates bileaflet mitral valve prolapse; bpm, beats per minute; ICD, implantable cardioverter‐defibrillator; LGE, late‐gadolinium enhancement; MR, mitral regurgitation; MV, mitral valve; MVP, mitral valve prolapse; OHCA, out‐of‐hospital cardiac arrest; NS, not specified; PM, papillary muscle; PVCs, premature ventricular complexes; QTc, corrected QT; SCD, sudden cardiac death; VAs, ventricular arrhythmias; VF, ventricular fibrillation; VT, ventricular tachycardia.
Significant result on multivariate analysis; significant univariable predictors are not presented.
Significant multivariate predictors of various outcomes include female sex and anterior mitral leaflet thickness for Lown grade ≥3 complex VAs, QTc dispersion and anterior mitral leaflet length for VT, moderate to severe MR for PVCs and VAs, degree of MVP and anterior mitral leaflet thickness for QT dispersion, and leaflet redundancy for SCD.
Incidence of SCD in MVP
We identified 3 prospective articles that described SCD events in patients with MVP (Table 6.18, 19, 20 .
Table 6.
Prospective Follow‐Up Studies in MVP With SCD Rates
| Study Author | Patients, No. | Mean Age, y | Females, No. | Mean Follow‐Up, y | SCD Events/100 000 Patient‐Y, No. |
|---|---|---|---|---|---|
| Nishimura18 | 237a | 44 | 142 | 6.2 | 408 |
| Düren19 | 300 | 42 | 164 | 6.2 | 219 |
| Zuppiroli20 | 316 | 42 | 220 | 8.5 | 112 |
MVP indicates mitral valve prolapse.
A total of 97 patients had redundant leaflets—all cases of sudden cardiac death (SCD) occurred in those with redundant leaflets.
Incidence of SCD ranged from 112 to 408 events per 100 000 person‐years, with an aggregate incidence of 217 events per 100 000 patient‐years (total 13 events in 5985.4 person‐years of follow‐up). One additional study described a pediatric cohort (mean age, 9.9 years) of patients with MVP with no SCD events during 814 person‐years of follow‐up.57
Discussion
This systematic review of all identified cases of cardiac arrest in patients with MVP demonstrates the following key features in patients with iMVP and SCD:
-
1
Clinical characteristics
-
a
Median age of 30 years (range 6–79 years) and 69% were female
-
b
A total of 47% of cases occurred during physiological or psychological stress
-
a
-
2
Cardiac electrophysiological findings
-
a
Frequent PVCs or VAs (92% on Holter monitoring)
-
b
VF is the primary rhythm (81%) in cardiac arrest and death
-
a
-
3
Cardiac imaging findings
-
a
Predominant (70%) bileaflet MVP
-
b
Moderate MR or less in 83%
-
a
-
4
Histopathological findings
-
a
Redundant leaflets in 99%
-
b
Abnormal chordae in 96%
-
a
-
5
Clinical predictors for SCD in MVP
-
a
Lacks robust evidence with heterogenous predictors and end points
-
b
Leaflet redundancy is the only independent predictor of SCD in patients with MVP
-
a
-
6
Estimated incidence of SCD in MVP is 217 events per 100 000 person‐years
Clinical Characteristics
The median age at time of cardiac arrest or SCD was 30 years, although this was 28 years in females and 39 years in males. The age‐sex distribution graph for the cases demonstrated a peak in female cases between 20 and 30 years consistent with previous data relating to iMVP and SCD.3, 42 Cases of MVP‐related cardiac arrest or SCD in males appeared evenly distributed throughout life.
There appeared to be a disproportionately large number of cases (47%) related to situations of stress (physical, emotional, driving, pregnancy, and in‐hospital). The association between increased adrenergic state and complex VAs may provide a plausible explanation as to why autonomic fluctuations may be important in the pathogenesis of iMVP related SCD.41
Cardiac Electrical Findings
From this large collection of MVP cases with cardiac arrest rhythm, VF appears to be primarily responsible for iMVP‐related SCD. Where documented, most were PVC triggered. Only 2 cases described cardiac arrest caused by asystole, with 1 patient having exercise‐induced asystole and 1 patient having a likely vagal reaction.58, 59 These findings support a primary arrhythmogenic cause of SCD in patients with iMVP.
Common ECG changes included the presence of inferolateral T‐wave inversion and PVCs on ECG and the presence of PVCs and VAs on Holter monitoring. However, despite the postulation that inferior T‐wave changes on ECG are associated with a potentially high‐risk MVP subtype,3, 34 prospective evidence is lacking. Similarly, despite reports of a high incidence of PVCs and VAs on Holter monitoring,60 these findings have not been prospectively correlated to SCD events in patients with MVP.
Inducible VAs on programmed ventricular stimulation does not appear to predict SCD events in patients with MVP.61 Two cases in this study reported programmed ventricular stimulation findings before SCD and both cases did not induce VAs.36, 62 Additionally, only 1 of 22 cases (5%) had sustained VT during programmed ventricular stimulation, suggesting that arrhythmia initiation is PVC triggered rather than re‐entrant scar related. As such, the role of electrophysiological extrastimuli testing in identifying a potential high‐risk MVP subtype may be limited.
Cardiac Imaging Findings
The presence of bileaflet prolapse has been associated with an increased rate of VAs and cardiac arrest.3, 45 This is consistent with our findings where a bileaflet phenotype was present in 70% of cases of SCD or cardiac arrest. The association between bileaflet prolapse, mitral annular disjunction, and VAs indicates that mitral apparatus abnormalities likely play a contributory role in the development of malignant VAs.63
Although prior studies suggest that severe MR is correlated with VAs,5 we found no association between them. Where degree of MR was reported, the majority (83%) of patients experienced cardiac arrest in the setting of nonsevere MR. Whether surgery on the MV may mitigate risk of cardiac arrest is also unclear. Patients who underwent MV surgery had variable results, including 2 cases that experienced recurrent VAs requiring defibrillator therapy post‐MV surgery.64 The lack of systematic reporting and long‐term follow‐up limits our interpretation.
Other cardiac imaging parameters that may be important include degree of redundancy,18 mitral annular dilatation,63 mitral annular disjunction,63 and anterior mitral leaflet thickness and length.42, 48 Unfortunately, few studies documented findings in regard to these parameters. Furthermore, although previous work has suggested that radiological myocardial fibrosis may be a trigger for complex VAs in MVP,4, 45 results from cardiac magnetic resonance imaging were only available in 2 studies, limiting interpretation. Studies that prospectively evaluate cardiac imaging parameters with systematic reporting of longitudinal outcomes are required.
Cardiac Structural Findings
Where reported, 99% of cases described mitral leaflet redundancy, and MV annulus diameter was dilated compared with population data.65 Anterior and posterior mitral leaflet length were also greater than otherwise expected.66 Abnormal chordal findings were present in 96% of cases. The combination of morphological valve distortion and chordal abnormalities are consistent with other autopsy studies of patients with MVP66, 67 and provide further support that mitral apparatus abnormalities have a contributory role in the development of SCD.
There were 30 cases where cardiac histopathological findings were described. Among these, 12 cases reported abnormal left ventricular histological changes, including 3 cases that specifically described histological abnormalities involving the papillary muscles. Left ventricular fibrosis, especially near the papillary muscles, is described in autopsy patients with MVP and may provide a substrate for the development of VAs.4, 68 These findings suggest that both diffuse and focal changes within the left ventricle occur in patients with MVP, which may act as a substrate for the development of VAs.
Findings in Non‐iMVP
As described, there was a subset of patients with SCD and MVP but also other cardiac abnormalities.
SCD is likely attributable to significant coronary artery disease, dilated or hypertrophic cardiomyopathy, Brugada syndrome, and arrhythmogenic right ventricular cardiomyopathy in cases with these coexistent conditions.
Other coexistent findings are more contentious. Anatomical findings such as mild left ventricular hypertrophy or cardiomegaly at autopsy have been described in relation to MVP69 and could indicate that pathological changes of the ventricle in otherwise “iMVP” is a contributor to SCD events. Additionally, 13 patients were taking antiarrhythmic medications. It is prudent to consider that while these medications in themselves may have proarrhythmic side effects, these medications were likely administered to treat preexisting VAs in the cases. Finally, findings of prolonged QTc may also reflect underlying repolarization abnormalities in patients with MVP, which has also been previously described.48, 51 .
Challenges in Predicting SCD in Patients With Isolated MVP
Studies investigating premortem predictors of SCD in MVP are limited. One prospective study demonstrated that leaflet redundancy was an independent predictor of SCD.18 Some controversy surrounds the risk of bileaflet MVP with 1 study suggesting that it was associated with appropriate implantable cardioverter‐defibrillator therapies,3 while another suggested that bileaflet MVP was associated with lower all‐cause mortality based on registry data.55
Premortem predictors of VAs are difficult to validate in the current collection of cases. Some predictors such as leaflet redundancy, bileaflet MVP, and inferolateral T‐wave inversion on ECG were only available in approximately half of the case reports, while degree of MR was available for about one quarter of cases. Other potential predictors such as catecholamine levels, late potentials, QT dispersion, anterior mitral leaflet thickness and length, mitral annular disjunction, presence of late‐gadolinium enhancement, and myocardial T1 time were either scarcely reported or not reported.
In addition, many studies have used VAs or repolarization abnormalities as surrogate end points for SCD because of the relatively low event rates of SCD. These end points, which include nonsustained ventricular tachycardia, Lown grade VAs of varying degrees, PVC frequency, exercise‐induced PVCs, presence of papillary muscle PVCs, PVC reduction post‐MV surgery, corrected QT interval, or QT dispersion, are yet to be validated as predictors of SCD in the MVP population.
The heterogeneous nature of these predictors and end points limits comparisons between studies. As such, despite the numerous cases reporting SCD or cardiac arrest in MVP, there is limited evidence that such outcomes can be reliably predicted.
Incidence of SCD in MVP
Our findings suggest that the overall incidence of SCD in MVP was 217 events per 100 000 person‐years based on 3 prospective studies, although the presence of leaflet redundancy may signal a higher risk cohort. Extrapolation of data from Nishimura et al18 suggests an approximate event rate of 998 per 100 000 person‐years in patients with evidence of leaflet redundancy.
Comparisons to population data are inherently limited (Figure 5). More recent population‐based studies indicate that the incidence of SCD in the general population has decreased from 94 to 97 events per 100 000 person‐years in the 1990s to 42 to 53 events per 100 000 person‐years in the 2000s,70, 71, 72, 73, 74 although advances in resuscitation methods may account for some of this difference. Framingham data (involving an older and more male‐predominant cohort) suggest that the SCD risk in the general population was ≈130 events per 100 000 person‐years during the 1980s,71 around the time of the 3 prospective studies.
Figure 5.
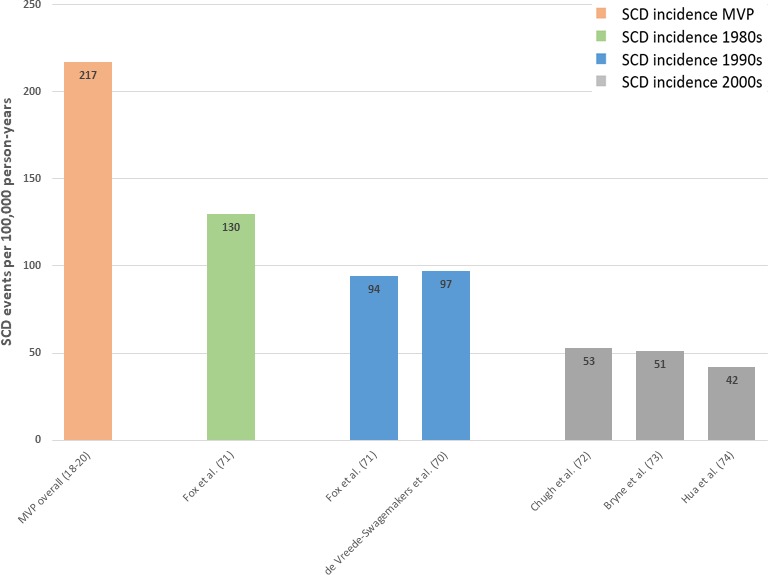
Sudden cardiac death (SCD) incidence in mitral valve prolapse (MVP) versus population studies.
Limitations
This is the largest systematic review of published cases of MVP and SCD or cardiac arrest. We sought to provide comprehensive insight into clinical, electrical, imaging, and histopathological characteristics. Our results highlight some significant challenges when attempting to characterize a potential high‐risk MVP subtype.
The cases that describe MVP and SCD or cardiac arrest span over 50 years. Our understanding of MVP has evolved significantly over that time. Changes in clinical medicine affect the reproducibility of various diagnostic tests, especially echocardiography for the diagnosis of MVP. Information regarding clinical, electrical, imaging, and histopathological characteristics were inconsistently described and are subject to reporting and publication bias. Notably, a lack of systematic reporting regarding these characteristics likely affected their prevalence within this collection of cases.
Further work is required to validate many of the current reported predictors. The disconnect between premortem predictors and available information from SCD cases limits our ability to determine whether these factors may be important in the development of SCD and cardiac arrest.
Finally, despite all the published literature hypothesizing that SCD in MVP is caused by malignant VAs, there are only 6 cases describing autopsy‐proven iMVP with documentation of cardiac arrest rhythm. Further correlations of cardiac arrest rhythm with pathological description is warranted.
Conclusions
Our systematic review indicates that iMVP and SCD predominantly affects young females. The MV leaflets are frequently redundant with bileaflet prolapse, associated chordal abnormalities, and nonsevere MR. Electrophysiological changes include frequent PVCs on Holter monitoring and VF as the predominant cardiac arrest rhythm. Current predictors for SCD events in iMVP lack robust evidence. To better understand the complex relationship between MVP and SCD, standardized reporting of clinical, electrophysiological, echocardiographic, and other cardiac imaging variables with documentation of long‐term outcomes is required.
Disclosures
Dr Han and Dr Lim report having received funding from Austin Medical Research Foundation. The remaining authors have no relevant disclosures to report.
Supporting information
Table S1. All Cases Included in the Study
Table S2. Predictors of VAs or SCD
(J Am Heart Assoc. 2018;7:e010584 DOI: 10.1161/JAHA.118.010584.)
References
- 1. Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical outcome of mitral‐valve prolapse. N Engl J Med. 1999;341:1–7. [DOI] [PubMed] [Google Scholar]
- 2. Hayek E, Gring CN, Griffin BP. Mitral valve prolapse. Lancet. 2005;365:507–518. [DOI] [PubMed] [Google Scholar]
- 3. Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez‐Sarano M, Cetta F, Cannon BC, Asirvatham SJ, Ackerman MJ. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out‐of‐hospital cardiac arrest. J Am Coll Cardiol. 2013;62:222–230. [DOI] [PubMed] [Google Scholar]
- 4. Basso C, Perrazolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A, Chiara Frigo A, Rigato I, Migliore F, Pilichou K, Bertaglia E, Cacciavillani L, Bauce B, Corrado D, Thiene G, Iliceto S. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:556–566. [DOI] [PubMed] [Google Scholar]
- 5. Kligfield P, Hochreiter C, Kramer H, Devereux RB, Niles N, Kramer‐Fox R, Borer JS. Complex arrhythmias in mitral regurgitation with and without mitral valve prolapse: contrast to arrhythmias in mitral valve prolapse without mitral regurgitation. Am J Cardiol. 1985;55:1545–1549. [DOI] [PubMed] [Google Scholar]
- 6. Barlow J, Pocock W, Marchand P, Denny M. The significance of late systolic murmurs. Am Heart J. 1963;66:443–452. [Google Scholar]
- 7. Davies M, Moore B, Braimbridge M. The floppy mitral valve. Study of incidence, pathology, and complications in surgical, necropsy, and forensic material. Br Heart J. 1978;40:468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chesler E, King RA, Edwards JE. The myxomatous mitral valve and sudden death. Circulation. 1983;67:632–639. [DOI] [PubMed] [Google Scholar]
- 9. Pocock WA, Bosman CK, Chesler E, Barlow JB, Edwards JE. Sudden death in primary mitral valve prolapse. Am Heart J. 1984;107:378–382. [DOI] [PubMed] [Google Scholar]
- 10. Scala‐Barnett DM, Donoghue E. Sudden death in mitral valve prolapse. J Forensic Sci. 1988;33:84–91. [PubMed] [Google Scholar]
- 11. Dollar AL, Roberts WC. Morphologic comparison of patients with mitral valve prolapse who died suddenly with patients who died from severe valvular dysfunction or other conditions. J Am Coll Cardiol. 1991;17:921–931. [DOI] [PubMed] [Google Scholar]
- 12. Kerber RE, Isaeff DM, Hancock EW. Echocardiographic patterns in patients with the syndrome of systolic click and late systolic murmur. N Engl J Med. 1971;284:691–693. [DOI] [PubMed] [Google Scholar]
- 13. Dillon JC, Haine CL, Chang S, Feigenbaum H. Use of echocardiography in patients with prolapsed mitral valve. Circulation. 1971;43:503–507. [DOI] [PubMed] [Google Scholar]
- 14. Morganroth J, Jones RH, Chen CC, Naito M. Two dimensional echocardiography in mitral, aortic and tricuspid valve prolapse: the clinical problem, cardiac nuclear imaging considerations and a proposed standard for diagnosis. Am J Cardiol. 1980;46:1164–1177. [DOI] [PubMed] [Google Scholar]
- 15. Levine RA, Handschumacher MD, Sanfilippo AJ, Hagege AA, Harrigan P, Marshall JE, Weyman AE. Three‐dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation. 1989;80:589–598. [DOI] [PubMed] [Google Scholar]
- 16. Savage DD, Garrison RJ, Devereux RB, Castelli WP, Anderson SJ, Levy D, McNamara PM, Stokes J, Kannel WB, Feinleib M. Mitral valve prolapse in the general population. I. Epidemiologic features: the Framingham study. Am Heart J. 1983;106:571–576. [DOI] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishimura RA, McGoon MD, Shub C, Miller FA Jr, Ilstrup DM, Jamil Tajik A. Echocardiographically documented mitral‐valve prolapse. N Engl J Med. 1985;313:1305–1309. [DOI] [PubMed] [Google Scholar]
- 19. Düren DR, Becker AE, Dunning AJ. Long‐term follow‐up of idiopathic mitral valve prolapse in 300 patients: a prospective study. J Am Coll Cardiol. 1988;11:42–47. [DOI] [PubMed] [Google Scholar]
- 20. Zuppiroli A, Rinaldi M, Kramer‐Fox R, Favilli S, Roman MJ, Devereux RB. Natural history of mitral valve prolapse. Am J Cardiol. 1995;75:1028–1032. [DOI] [PubMed] [Google Scholar]
- 21. Frassati D, Tabib A, Lachaux B, Giloux N, Daléry J, Vittori F, Charvet D, Barel C, Bui‐Xuan B, Mégard R, Jenoudet L‐P, Descotes J, Vial T, Timour Q. Hidden cardiac lesions and psychotropic drugs as a possible cause of sudden death in psychiatric patients: a report of 14 cases and review of the literature. Can J Psychiatry. 2004;49:100–105. [DOI] [PubMed] [Google Scholar]
- 22. Cannon ME, Cooke CT, McCarthy JS. Caffeine‐induced cardiac arrhythmia: an unrecognised danger of healthfood products. Med J Aust. 2001;174:520–521. [DOI] [PubMed] [Google Scholar]
- 23. Winkle RA, Lopes MG, Popp RL, Hancock EW. Life‐threatening arrhythmias in the mitral valve prolapse syndrome. Am J Med. 1976;60:961–967. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JR. Automatic burst extrastimulus pacemaker to treat recurrent ventricular tachycardia in a patient with mitral valve prolapse: more than 2,000 documented successful tachycardia terminations. J Am Coll Cardiol. 1986;8:446–450. [DOI] [PubMed] [Google Scholar]
- 25. Rajani AR, Murugesan V, Baslaib FO, Rafiq MA. Mitral valve prolapse and electrolyte abnormality: a dangerous combination for ventricular arrhythmias. BMJ Case Rep. 2014;2014:bcr2014205055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin LT, Tsui KH, Chang R, Cheng JT, Huang BS, Wang PH. Management of recurrent and refractory ventricular tachycardia in pregnancy. Taiwan J Obstet Gynecol. 2015;54:319–321. [DOI] [PubMed] [Google Scholar]
- 27. Ritchie JL, Hammermeister KE, Kennedy JW. Refractory ventricular tachycardia and fibrillation in a patient with the prolapsing mitral leaflet syndrome: successful control with overdrive pacing. Am J Cardiol. 1976;37:314–316. [DOI] [PubMed] [Google Scholar]
- 28. Bennett KR. Torsade de pointes and mitral valve prolapse. Am J Cardiol. 1980;45:715. [DOI] [PubMed] [Google Scholar]
- 29. Martini B, Basso C, Thiene G. Sudden death in mitral valve prolapse with Holter monitoring‐documented ventricular fibrillation: evidence of coexisting arrhythmogenic right ventricular cardiomyopathy. Int J Cardiol. 1995;49:274–278. [DOI] [PubMed] [Google Scholar]
- 30. Abbadi DR, Purbey R, Poornima IG. Mitral valve repair is an effective treatment for ventricular arrhythmias in mitral valve prolapse syndrome. Int J Cardiol. 2014;177:e16–e18. [DOI] [PubMed] [Google Scholar]
- 31. Saha T, Norris R, Luebbert J. Recurrent premature ventricular contraction–induced ventricular fibrillation and resuscitated sudden death in a 26‐year‐old pregnant woman with bileaflet mitral valve prolapse. HeartRhythm Case Rep. 2018;4:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trent J, Adelman A, Wigle E, Silver M. Morphology of a prolapsed posterior mitral valve leaflet. Am Heart J. 1970;79:539–543. [DOI] [PubMed] [Google Scholar]
- 33. Kleid JJ. Sudden death and the floppy mitral valve syndrome. Angiology. 1976;27:734–737. [DOI] [PubMed] [Google Scholar]
- 34. Jeresaty RM. Sudden death in the mitral valve prolapse‐click syndrome. Am J Cardiol. 1976;37:317–318. [DOI] [PubMed] [Google Scholar]
- 35. Salmela PI, Ikäheimo M, Juustila H. Fatal ventricular fibrillation after treatment with digoxin in a 27‐year‐old man with mitral leaflet prolapse syndrome. Br Heart J. 1981;46:338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boudoulas H, Schaal SF, Stang JM, Fontana ME, Kolibash AJ, Wooley CF. Mitral valve prolapse: cardiac arrest with long‐term survivial. Int J Cardiol. 1990;26:37–44. [DOI] [PubMed] [Google Scholar]
- 37. Franchitto N, Bounes V, Telmon N, Rougé D. Mitral valve prolapse and out‐of‐hospital sudden death: a case report and literature review. Med Sci Law. 2010;50:164–167. [DOI] [PubMed] [Google Scholar]
- 38. Nolte KB. Sudden cardiac death owing to pseudoxanthoma elasticum: a case report. Hum Pathol. 2000;31:1002–1004. [DOI] [PubMed] [Google Scholar]
- 39. Gaffney FA, Karlsson ES, Campbell W, Schutte JE, Nixon J, Willerson JT, Blomqvist CG. Autonomic dysfunction in women with mitral valve prolapse syndrome. Circulation. 1979;59:894–901. [DOI] [PubMed] [Google Scholar]
- 40. Puddu PE, Pasternac A, Tubau JF, Król R, Farley L, de Champlain J. QT interval prolongation and increased plasma catecholamine levels in patients with mitral valve prolapse. Am Heart J. 1983;105:422–428. [DOI] [PubMed] [Google Scholar]
- 41. Śniez̊ek‐Maciejewska M, Dubiel J, Piwowarska W, Mroczek‐Czernecka D, Mazurek S, Jaśkiewicz J, Kitliński M. Ventricular arrhythmias and the autonomic tone in patients with mitral valve prolapse. Clin Cardiol. 1992;15:720–724. [DOI] [PubMed] [Google Scholar]
- 42. Zuppiroli A, Mori F, Favilli S, Barchielli A, Corti G, Montereggi A, Dolara A. Arrhythmias in mitral valve prolapse: relation to anterior mitral leaflet thickening, clinical variables, and color Doppler echocardiographic parameters. Am Heart J. 1994;128:919–927. [DOI] [PubMed] [Google Scholar]
- 43. Babuty D, Cosnay P, Breuillac J, Charniot J, Delhomme C, Fauchier L, Fauchier J. Ventricular arrhythmia factors in mitral valve prolapse. Pacing Clin Electrophysiol. 1994;17:1090–1099. [DOI] [PubMed] [Google Scholar]
- 44. Naksuk N, Syed FF, Krittanawong C, Anderson MJ, Ebrille E, DeSimone CV, Vaidya VR, Ponamgi SP, Suri RM, Ackerman MJ, Nkomo VT, Asirvatham SJ, Noseworthy PA. The effect of mitral valve surgery on ventricular arrhythmia in patients with bileaflet mitral valve prolapse. Indian Pacing Electrophysiol J. 2016;16:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fulton BL, Liang JJ, Enriquez A, Garcia FC, Supple GE, Riley MP, Schaller RD, Dixit S, Callans DJ, Marchlinski FE, Han Y. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J Cardiovasc Electrophysiol. 2018;29:146–153. [DOI] [PubMed] [Google Scholar]
- 46. Campbell R, Godman M, Fiddler G, Marquis R, Julian D. Ventricular arrhythmias in syndrome of balloon deformity of mitral valve. Definition of possible high risk group. Heart. 1976;38:1053–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bobkowski W, Siwińska A, Zachwieja J, Mroziński B, Rzeźnik‐Bieniaszewska A, Maciejewski J. A prospective study to determine the significance of ventricular late potentials in children with mitral valvar prolapse. Cardiol Young. 2002;12:333–338. [DOI] [PubMed] [Google Scholar]
- 48. Akcay M, Yuce M, Pala S, Akcakoyun M, Ergelen M, Kargin R, Emiroglu Y, Ozdemir N, Kaymaz C, Ozkan M. Anterior mitral valve length is associated with ventricular tachycardia in patients with classical mitral valve prolapse. Pacing Clin Electrophysiol. 2010;33:1224–1230. [DOI] [PubMed] [Google Scholar]
- 49. Shah AA, Quinones MA, Waggoner AD, Barndt R, Miller RR. Pulsed Doppler echocardiographic detection of mitral regurgitation in mitral valve prolapse: correlation with cardiac arrhythmias. Cathet Cardiovasc Diagn. 1982;8:437–444. [DOI] [PubMed] [Google Scholar]
- 50. Sanfilippo AJ, Abdollah H, Burggraf GW. Quantitation and significance of systolic mitral leaflet displacement in mitral valve prolapse. Am J Cardiol. 1989;64:1349–1355. [DOI] [PubMed] [Google Scholar]
- 51. Zouridakis E, Parthenakis F, Kochiadakis G, Kanoupakis E, Vardas P. QT dispersion in patients with mitral valve prolapse is related to the echocardiographic degree of the prolapse and mitral leaflet thickness. Europace. 2001;3:292–298. [DOI] [PubMed] [Google Scholar]
- 52. Turker Y, Ozaydin M, Acar G, Ozgul M, Hoscan Y, Varol E, Dogan A, Erdogan D, Yucel H. Predictors of ventricular arrhythmias in patients with mitral valve prolapse. Int J Cardiovasc Imaging. 2010;26:139–145. [DOI] [PubMed] [Google Scholar]
- 53. Carmo P, Andrade MJ, Aguiar C, Rodrigues R, Gouveia R, Silva JA. Mitral annular disjunction in myxomatous mitral valve disease: a relevant abnormality recognizable by transthoracic echocardiography. Cardiovas Ultrasound. 2010;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, Kissinger KV, Zimetbaum PJ, Manning WJ, Yeon SB. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging. 2008;1:294–303. [DOI] [PubMed] [Google Scholar]
- 55. Nordhues BD, Siontis KC, Scott CG, Nkomo VT, Ackerman MJ, Asirvatham SJ, Noseworthy PA. Bileaflet mitral valve prolapse and risk of ventricular dysrhythmias and death. J Cardiovasc Electrophysiol. 2016;27:463–468. [DOI] [PubMed] [Google Scholar]
- 56. Bui AH, Roujol S, Foppa M, Kissinger KV, Goddu B, Hauser TH, Zimetbaum PJ, Ngo LH, Manning WJ, Nezafat R, Delling FN. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart. 2017;103:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bisset GS, Schwartz DC, Meyer RA, James FW, Kaplan S. Clinical spectrum and long‐term follow‐up of isolated mitral valve prolapse in 119 children. Circulation. 1980;62:423–429. [DOI] [PubMed] [Google Scholar]
- 58. Goldhammer E, Malouf S, Hassan A, Abinader E. Ventricular asystole and syncope in mitral valve prolapse: case report. J Cardiopulm Rehabil Prev. 1988;8:324–325. [Google Scholar]
- 59. Abraham ZA, Lees DE. Two cardiac arrests after needle punctures in a patient with mitral valve prolapse: psychogenic? Anest Analg. 1989;69:126–128. [PubMed] [Google Scholar]
- 60. Kulan K, Komsuoğlu B, Tuncer C, Kulan C. Significance of QT dispersion on ventricular arrhythmias in mitral valve prolapse. Int J Cardiol. 1996;54:251–257. [DOI] [PubMed] [Google Scholar]
- 61. Rosenthal ME, Hamer A, Gang ES, Oseran DS, Mandel WJ, Peter T. The yield of programmed ventricular stimulation in mitral valve prolapse patients with ventricular arrhythmias. Am Heart J. 1985;110:970–976. [DOI] [PubMed] [Google Scholar]
- 62. Morady F, Scheinman MM, Hess DS, Chen R, Stanger P. Clinical characteristics and results of electrophysiologic testing in young adults with ventricular tachycardia or ventricular fibrillation. Am Heart J. 1983;106:1306–1314. [DOI] [PubMed] [Google Scholar]
- 63. Marra MP, Basso C, De Lazzari M, Rizzo S, Cipriani A, Giorgi B, Lacognata C, Rigato I, Migliore F, Pilichou K. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovas Imaging. 2016;9:e005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vaidya VR, DeSimone CV, Damle N, Naksuk N, Syed FF, Ackerman MJ, Ponamgi SP, Nkomo VT, Suri RM, Noseworthy PA, Asirvatham SJ. Reduction in malignant ventricular arrhythmia and appropriate shocks following surgical correction of bileaflet mitral valve prolapse. J Interv Card Electrophysiol. 2016;46:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kitzman DW, Scholz DG, Hagen PT, Ilstrup DM, Edwards WD. Age‐related changes in normal human hearts during the first 10 decades of life. Part II (maturity): a quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988;63:137–146. [DOI] [PubMed] [Google Scholar]
- 66. Farb A, Tang AL, Atkinson JB, McCarthy WF, Virmani R. Comparison of cardiac findings in patients with mitral valve prolapse who die suddenly to those who have congestive heart failure from mitral regurgitation and to those with fatal noncardiac conditions. Am J Cardiol. 1992;70:234–239. [DOI] [PubMed] [Google Scholar]
- 67. Marchand P, Barlow JB, Du Plessis LA, Webster I. Mitral regurgitation with rupture of normal chordae tendineae. Br Heart J. 1966;28:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Burke AP, Farb A, Tang A, Smialek J, Virmani R. Fibromuscular dysplasia of small coronary arteries and fibrosis in the basilar ventricular septum in mitral valve prolapse. Am Heart J. 1997;134:282–291. [DOI] [PubMed] [Google Scholar]
- 69. Chugh SS, Kelly KL, Titus JL. Sudden cardiac death with apparently normal heart. Circulation. 2000;102:649–654. [DOI] [PubMed] [Google Scholar]
- 70. de Vreede‐Swagemakers JJ, Gorgels AP, Dubois‐Arbouw WI, Van Ree JW, Daemen MJ, Houben LG, Wellens HJ. Out‐of‐hospital cardiac arrest in the 1990s: a population‐based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30:1500–1505. [DOI] [PubMed] [Google Scholar]
- 71. Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110:522–527. [DOI] [PubMed] [Google Scholar]
- 72. Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate‐based review in a large US community. J Am Coll Cardiol. 2004;44:1268–1275. [DOI] [PubMed] [Google Scholar]
- 73. Byrne R, Constant O, Smyth Y, Callagy G, Nash P, Daly K, Crowley J. Multiple source surveillance incidence and aetiology of out‐of‐hospital sudden cardiac death in a rural population in the West of Ireland. Eur Heart J. 2008;29:1418–1423. [DOI] [PubMed] [Google Scholar]
- 74. Hua W, Zhang LF, Wu YF, Liu XQ, Guo DS, Zhou HL, Gou ZP, Zhao LC, Niu HX, Chen KP, Mai JZ, Chu LN, Zhang S. Incidence of sudden cardiac death in China: analysis of 4 regional populations. J Am Coll Cardiol. 2009;54:1110–1118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. All Cases Included in the Study
Table S2. Predictors of VAs or SCD


