Abstract
Background
Acute rheumatic fever (ARF) and rheumatic heart disease cause substantial burdens worldwide. Long‐term antibiotic secondary prophylaxis is used to prevent disease progression, but evidence for benefits of different adherence levels is limited. Using data from northern Australia, we identified factors associated with adherence, and the association between adherence and ARF recurrence, progression to rheumatic heart disease, worsening or improvement of rheumatic heart disease, and mortality.
Methods and Results
Factors associated with adherence (percent of doses administered) were analyzed using logistic regression. Nested case–control and case–crossover designs were used to investigate associations with clinical outcomes; conditional logistic regression was used to estimate odds ratios (OR) with 95% CIs Adherence estimates (7728) were analyzed. Being female, younger, having more‐severe disease, and living remotely were associated with higher adherence. Alcohol misuse was associated with lower adherence. The risk of ARF recurrence did not decrease until ≈40% of doses had been administered. Receiving <80% was associated with a 4‐fold increase in the odds of ARF recurrence (case–control OR: 4.00 [95% CI: 1.7–9.29], case–crossover OR: 3.31 [95% CI: 1.09–10.07]) and appeared to be associated with increased all‐cause mortality (case–control OR: 1.90 [95% CI: 0.89–4.06]; case–crossover OR 1.91 [95% CI: 0.51–7.12]).
Conclusions
We show for the first time that increased adherence to penicillin prophylaxis is associated with reduced ARF recurrence, and a likely reduction in mortality, in our setting. These findings can motivate patients to receive doses since even relatively low adherence can be beneficial, and additional doses further reduce adverse clinical outcomes.
Keywords: acute rheumatic fever, adherence, Australian Indigenous, rheumatic heart disease, secondary prophylaxis
Subject Categories: Secondary Prevention, Quality and Outcomes
Clinical Perspective
What Is New?
This is the first comprehensive analysis of the effectiveness of penicillin prophylaxis for acute rheumatic fever at a population level.
Among 1610 people prescribed penicillin prophylaxis, increased adherence was associated with reduced risk of acute rheumatic fever recurrence and death: for every 10% increase in adherence, the odds of acute rheumatic fever recurrence reduced by 17% and the odds of death reduced by ≈12%.
At the individual level, the likelihood of acute rheumatic fever recurrence decreased once 40% of doses were administered and continued to decrease with each additional dose.
What Are the Clinical Implications?
The evidence that increased adherence prevents acute rheumatic fever recurrence and death should be a powerful motivational tool to encourage health professionals and clients to maximize adherence.
The results provide a rationale for a particular focus on ensuring all clients receive at least 40% of their prescribed doses.
At the system level, our findings support the programmatic use of ≥80% as an adherence target, since greatest benefit was achieved at this level of adherence.
Introduction
Acute rheumatic fever (ARF) is the consequence of an abnormal immune response to an infection by β‐hemolytic Group A streptococcus (GAS) bacteria. There are several possible manifestations of ARF including fever and sore joints; the valves of the heart may also be damaged and residual scarring is called rheumatic heart disease (RHD). ARF and RHD continue to cause significant morbidity and premature mortality in disadvantaged populations; in 2015 there were ≈33 million people living with RHD worldwide.1
Studies conducted in the 1930s showed for the first time that sulphonamide antibiotics could prevent ARF, and subsequent studies found that penicillin was superior to sulphonamides.2 Penicillin eradicated GAS, whereas sulphonamides did not prevent carriage, and the dosing regimens were simpler, which improved adherence.2 The first penicillin formulations studied involved multiple daily doses or several injections.2, 3 Benzathine penicillin G (BPG) was subsequently proven to be the most effective formulation for eradicating GAS and only 1 intramuscular dose was required.3 BPG remains the antibiotic of choice for primary and secondary prophylaxis of ARF. In accordance with guidelines published by the World Health Organization,4 in many settings efforts to control RHD focus on providing a long‐term regimen of regular injections of BPG to people with a history of ARF or RHD (referred to as secondary prophylaxis [SP]) in order to prevent recurrent ARF. The painful nature of the injections, the protracted duration of the regimen (at least 10 years in Australia), and limited resources in many settings where ARF and RHD are prevalent mean delivering SP is challenging.5 Increased adherence to BPG has been reported to reduce the likelihood of recurrent ARF in several populations,6, 7, 8, 9, 10, 11, 12, 13, 14 but there has been limited investigation of the effect of different levels of adherence, the methods used to calculate adherence are not always clearly described, self‐reported adherence is often used, and there is no rigorous evidence that the widely used target of ≥80% of doses is optimal or superior to other thresholds. To improve the effectiveness of RHD control programs internationally, a robust analysis of the association between adherence to SP and clinical outcomes is required.
This study used data from Australia's Northern Territory (NT) to determine the association between adherence and clinical outcomes for people living with ARF or RHD. To strengthen efforts to increase adherence to SP in the NT, where rates of RHD in the Indigenous population remain among the highest in the world,15 factors associated with adherence were also explored.
Methods
The analytic methods will be made available to other researchers for purposes of reproducing the results or replicating the procedure. The data that support the findings of this study may be requested from the corresponding author and requests will be considered by a data governance panel.
Study Design and Setting
A retrospective cohort design was used to investigate factors associated with adherence. Nested case–control and case–crossover designs were used to explore the association between clinical outcomes and adherence.
Data from the NT RHD Register (the Register) were linked to hospital admissions from the NT Hospital Admissions database. The Register data included diagnoses of ARF and RHD and medical reviews (including cardiologist reviews and echocardiogram reports) and secondary prophylaxis adherence data. ARF has been notifiable in the NT since 199516 and all suspected and confirmed episodes must be reported and are recorded in the Register. In the NT, BPG is predominantly administered by health staff at primary health centers; administration dates are shared with the NT RHD Control Program and are recorded in the Register. The study period was February 1, 2007 to December 31, 2013. Another report using this data set has been published previously.17 Health care, including BPG injections, is provided to Indigenous patients via Aboriginal Health Services. Each remote community is serviced by 1 clinic, usually within walking distance of all houses in the community, and urban settings have 1 or more dedicated Aboriginal Health Services. Some clinics provide an outreach service (which can change from 1 month to another depending on staff availability and preference), but the majority of clinics require patients to attend in person to receive injections. In the latter instance, a clinic bus is usually provided to pick up patients for their injections.
Validation of Register Data
Dates of death were validated using data from the Client Master Index database (the most reliable record of death data in the NT). Diagnoses of ARF and RHD were examined for consistency and concordance with national guidelines, and diagnoses made during the study period were compared with hospital admissions with relevant International Classification of Diseases Tenth revision, Australian Modification (ICD‐10‐AM) codes (I00–I02 and I05–I09). Inconsistencies between the disease classification, which indicates the severity of RHD, and the heart valve lesion status were identified and clarified using original data from cardiologist letters, echocardiogram reports, and angiogram reports in the NT Cardiac database (NT Cardiac provides the majority of cardiologist reviews and cardiac tests in the NT). The validity of secondary prophylaxis data was investigated by identifying internal inconsistencies and comparing a random sample of dose dates for the year 2008 with the dates recorded in the primary healthcare database used by NT Government primary health centers and 1 community‐controlled primary health service. Inconsistencies were clarified by NT RHD Control Program staff. The validity of more recent dose dates had already been confirmed.18 Duplicate dose dates for an individual were removed.
Inclusion Criteria
The study population was people living in the NT with a history of ARF or RHD who were prescribed BPG SP.
Measurement of Adherence
Percent adherence was used to measure the delivery of SP. Percent adherence is the basis for monitoring adherence recommended in Australia's national guideline for RHD control, which also sets a recommended Key Performance Indicator for RHD Control Programs based on the proportion of individuals receiving ≥80% of scheduled doses (RHDAustralia [ARF/RHD writing group], 2012). There is no agreed minimum time frame for adherence calculations for BPG SP, so in the interest of statistical robustness, adherence calculations were restricted to people prescribed at least 6 doses of BPG (based on a 4‐weekly regimen; this equates to 168 days).
Percent adherence was estimated using the formula:
The standard regimen in Australia is 4‐weekly BPG,19 so the number of doses recommended was calculated by dividing the number of days that BPG was prescribed by 28. Three‐weekly dosing may be recommended in specific circumstances, but during the study period <1% of patients were prescribed 3‐weekly BPG. Continuous and dichotomized measures of adherence were analyzed.
Definitions of Adherence
For the analysis of factors associated with adherence, adherence was defined as ≥80% BPG doses (in accordance with the Australian guideline). For the analysis of clinical outcomes, multiple definitions of adherence were examined: ≥90% BPG doses, ≥80%, ≥70%, ≥60%, and ≥50%. Additional definitions of ≥40% and ≥30% were investigated for ARF recurrences.
Factors Associated With Adherence
Associations between adherence and patient demographics, disease severity, SP regimen, and comorbidities—separately or compiled in the Charlson Comorbidity Index, which includes 14 comorbidities—were investigated. Comorbidities that were examined separately included diabetes mellitus, and others not included in the Charlson Comorbidity Index: hypertension, intentional self‐harm, depression, obesity, experience of assault, hazardous alcohol use, and other drug misuse.
When information on the regimen frequency was unavailable, it was assumed the person was prescribed 4‐weekly BPG. Individual percent adherence was calculated for each calendar year. Hospital admissions from 2002 until 2013 were used to calculate the Charlson Comorbidity Index.20
Clinical Outcomes
Clinical outcomes that occurred while the person was prescribed SP were investigated: ARF recurrence (definite ARF only), progression from ARF to RHD, progression to severe RHD, improvement to mild RHD, and death. Recurrent ARF were episodes at least 90 days after any recorded previous episode.19 Progression from ARF to RHD was defined as a diagnosis of RHD at least 168 days (six 4‐weekly dosing intervals) after a first diagnosis of ARF; both diagnoses needed to have occurred during the study period. This interval was chosen to reduce the likelihood that RHD was present at the time of the first diagnosis of ARF; the timing of RHD diagnosis is dependent on the availability of echocardiography services and cardiologists, and there can be a lag between ARF and RHD diagnosis dates even when the RHD was present at the time of the ARF episode. The Australian guidelines for the prevention and management of ARF and RHD define mild, moderate, and severe categories of RHD based on echocardiographic and clinical features.19 Echocardiograms and cardiologist reviews were used to identify changes in disease severity. Progression to severe RHD was the diagnosis of severe RHD after a prior diagnosis of mild RHD; people who improved to mild RHD had a diagnosis of severe RHD followed by a diagnosis of mild RHD. Both the mild and severe diagnoses needed to occur during the study period while the person was prescribed SP. Because of the relative subjectivity of echocardiography, changes from mild to moderate disease or vice versa, or moderate to severe disease or vice versa, were not examined. Deaths from all causes were eligible for inclusion in the case–control and case–crossover analyses.
Statistical Analyses
Factors associated with adherence
Descriptive statistics were used to describe the demographic characteristics and to summarize annual adherence rates for the entire cohort. The proportion of people who received ≥80% doses disaggregated by each factor was calculated. Multivariable logistic regression with generalized estimating equations was used to identify factors associated with being adherent; all variables were included in the analysis (sex, age group, address, disease severity, time since diagnosis, SP regimen, year, any Charlson Comorbidity Index morbidity, type 2 diabetes mellitus, hypertension, intentional self‐harm, depression, obesity, experience of assault, hazardous alcohol use, and other drug misuse). Adjusted odds ratios (AOR), 95% CIs, and P values are reported.
Clinical outcomes—case–control analysis
Adherence among individuals with the outcome of interest (ARF recurrence, change in RHD severity, or death) was compared with adherence among controls without the outcome of interest. Confounding was limited by randomly matching each case to at least 1 control based on ethnicity, sex, age group, and region using Stata routine sttoc. Two controls were matched to each case for ARF recurrences and progression from ARF to RHD, 3 controls per case for deaths. Descriptive statistics were used to describe the characteristics of the cases. Median adherence and the proportion of people receiving ≥80% doses were calculated for cases and controls. Adherence was calculated for the period considered most likely to have contributed to the outcome, taking into consideration the minimum time frame required for a reliable estimate of adherence; the timing and duration of the periods were decided in consultation with experienced physicians and researchers. Table 1 shows adherence time frames for the case–control analysis.
Table 1.
Time Frames for Adherence in the Case–Control Analysis
| Outcome | Adherence Time Frame |
|---|---|
| ARF recurrence | 12 mo before onset of recurrence |
| Progression from ARF to RHD | From onset of first ARF episode to RHD diagnosis |
| Progression from mild to severe RHD | From most recent review with mild classification to first severe classification |
| Improvement from severe to mild RHD | From most recent review with severe classification to first mild classification |
| Death | 12 mo before death |
ARF indicates acute rheumatic fever; RHD, rheumatic heart disease.
For each clinical outcome, separate univariate linear regressions were conducted to identify associations with the continuous measure of percent adherence, and odds ratios (OR) with 95% CIs and P values were calculated. Possible nonlinear effects of the influence of adherence on clinical outcomes were estimated using fractional polynomials in univariate linear regression models.21 Nonparametric receiver operating characteristics curves plotting sensitivity against 1‐specificity were used to determine how well continuous percent adherence predicted each outcome of interest. Receiver operating characteristics curves can be used to depict the performance of diagnostic tests; the area under the receiver operating characteristics curve (AUC) is a measure of both the sensitivity and specificity and represents the overall performance of the test. The AUC ranges from 0 to 1; the closer the value is to 1, the stronger the performance of the test, and an AUC of 0.5 indicates no better than chance prediction. The AUC was used to indicate the strength of adherence to SP as a predictor of clinical outcomes. Adherence was not normally distributed so the AUC was calculated using nonparametric methods, namely, numerical integration with the trapezoidal rule.
Univariate conditional logistic regression was used to examine associations between dichotomized measures of adherence and clinical outcomes. Separate analyses were completed for each of the 5 definitions of adherence, and ORs with 95% CIs and P values were calculated. Population attributable fractions (PAF) with 95% CIs were calculated using Stata routine punafcc to predict the impact on the number of observed outcomes if the whole sample had reached the various definitions of adherence. The PAFs are reported in graphs.
Clinical outcomes—case–crossover analysis
In the case–crossover study, each person who experienced the outcome of interest acted as their own control: their adherence immediately before the event was compared with their adherence during other periods during which no event of interest occurred. The case–crossover design controls for relatively stable patient characteristics that were not captured by the available data set (such as education level and living conditions), and no additional matching or controlling for confounding is required. Furthermore, vulnerability to ARF after GAS infection is in part weakly genetically determined22; confounding by genetic variability was addressed by the case–crossover design. Given the greater level of control of confounding, the case–crossover results were thought to be less likely to be affected by bias. However, the sample sizes were much smaller in the case–crossover analyses. Descriptive statistics were used to describe the characteristics of the sample. Median adherence and the proportion of people receiving ≥80% doses were calculated for the case window and each of the control windows for each clinical outcome. This study is believed to be the first to apply the case–crossover design to the investigation of the association between adherence to SP and outcomes, and no literature on case and control windows was identified. Therefore, the case window (the period of adherence deemed most relevant to the development of the outcome) and the control windows (periods of adherence considered unrelated to the development of the outcome) were decided on in consultation with physicians and researchers with considerable experience in ARF and RHD. In addition to clinical relevance, the duration of the windows needed to enable a reliable estimate of adherence to be made. The case–crossover design could not be applied to progression from ARF to RHD because there were no appropriate control windows—the case window is from initial diagnosis of ARF until first diagnosis of RHD; control windows preceding the case window are not possible, since SP would not have been prescribed before the first diagnosis of ARF, and control windows after the diagnosis of RHD are not possible since the person is no longer at risk of developing RHD. The number of control windows was limited by the period of available data. Figure 1 shows the timing and duration of the case and control windows for each outcome.
Figure 1.
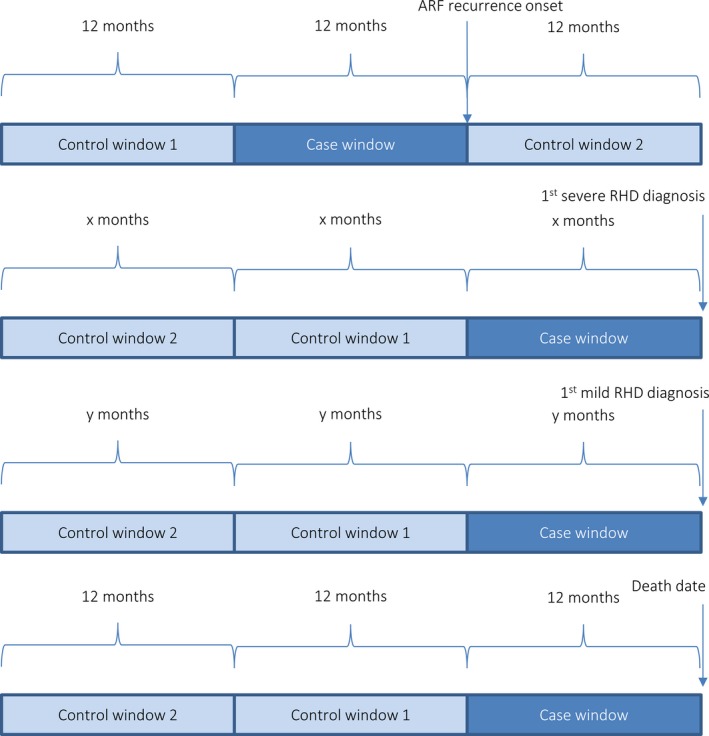
Case and control windows for case–crossover design. ARF indicates acute rheumatic fever; RHD, rheumatic heart disease.
Associations between continuous measures of percent adherence and clinical outcomes were explored using univariate linear regression and ORs with 95% CIs, and P values are reported for increases in adherence of 10 percentage points. As with the case–control analysis, univariate conditional logistic regression was used to examine associations between dichotomized measures of adherence and each clinical outcome; ORs with 95% CIs and P values were calculated. PAFs and 95% CIs were calculated using the same Stata routine (punafcc).
Analyses were conducted using Stata/IC 14.2 (StataCorp, College Station, TX); results were deemed significant at P<0.05.
This study was granted ethical approval by the Northern Territory Department of Health and Menzies School of Health Research human research ethics committee (ref: 2013‐2123); no informed consent was required. Access to identified patient data was granted for data validation.
Results
Factors Associated With Adherence
There were 1610 people who contributed 7888 annual estimates of adherence (median number of annual estimates per person: 3, total years follow‐up: 7536). Females accounted for 63% of the observations; 98% of the observations were for Indigenous Australians, and the median age was 24 years (range 3–79). Between 2007 and 2013, annual adherence ranged from 0% to 100% and median adherence was 59% (interquartile range: 31%–78%). See Table 2 for characteristics of the cohort.
Table 2.
Characteristics of Cohort for Analysis of Factors Associated With Adherence
| Factor | Categories | Estimates of Annual Adherence, n (%) |
|---|---|---|
| Ethnicity | Indigenous* | 7728 (98) |
| Not indigenous | 160 (2) | |
| Patient demographics (indigenous only) | ||
| Age | Children (0–14 y) | 1336 (17) |
| Adolescents (15–21 y) | 2008 (26) | |
| Adults (22–40 y) | 3444 (45) | |
| Older people (41 y and older) | 940 (12) | |
| Sex | Male | 2895 (37) |
| Female | 4833 (63) | |
| Residential area† | Urban | 1151 (15) |
| Not urban | 6577 (85) | |
| Disease and treatment characteristics (ARF and RHD) | ||
| Disease severity (at beginning of each y) | Severe RHD | 1805 (23) |
| Moderate RHD | 1391 (18) | |
| Mild RHD | 1804 (23) | |
| ARF only (no RHD) | 2728 (35) | |
| SP regimen | 3‐wkly BPG | 66 (1) |
| 4‐wkly BPG | 7662 (99) | |
| Time since first diagnosis‡ | Less than 1 y | 581 (8) |
| At least 1 y | 7147 (92) | |
| Comorbidities | ||
| Presence of a comorbidity§ | 2143 (28) | |
| Type 2 diabetes mellitus | 173 (3) | |
| Hypertension | 872 (11) | |
| Obesity | 259 (3) | |
| Hazardous alcohol use | 1272 (16) | |
| Other drug misuse | 336 (4) | |
| Depression | 193 (3) | |
| Intentional self‐harm | 316 (4) | |
| Experience of assault | 1184 (15) | |
| Calendar y | ||
| 2007 | 1033 (13) | |
| 2008 | 1053 (14) | |
| 2009 | 1078 (14) | |
| 2010 | 1096 (14) | |
| 2011 | 1120 (14) | |
| 2012 | 1147 (15) | |
| 2013 | 1201 (16) | |
ARF indicates acute rheumatic fever; BPG, benzathine penicillin G; RHD, rheumatic heart disease; SP, secondary prophylaxis.
Indigenous refers to people who identify as Aboriginal and/or Torres Strait Islander.
Urban residential areas are “Darwin urban” and “Alice Springs urban” as defined by the NT Department of Health districts.
In the middle of the calendar year.
Charlson Comorbidity Index ≥1.
The data indicate that Indigenous Australians were more adherent: 23% received ≥80% of doses, compared with 12% for people of other ethnicities (OR: 2.11 [95% CI: 0.84–5.36], P=0.113). To increase the relevance of findings to the majority of patients on SP in the NT, people of other ethnicities were removed from subsequent analyses, leaving 7728 observations.
Being female, living outside urban areas, and being obese were significantly associated with increased adherence. Females were more likely to be adherent (receive ≥80% doses) than males (AOR: 1.21 [95% CI: 1.02–1.44], P=0.030). People living outside urban areas were more likely to be adherent than those living in urban areas (AOR: 1.46 [95% CI: 1.13–1.88], P=0.004) and those who were obese were more likely to be adherent than those who were not (AOR: 1.71 [95% CI: 1.11–2.63], P=0.015).
Being older, having less severe disease, increasing time since first diagnosis, experience of assault, and hazardous alcohol use were predictors of lower adherence. Adolescents and adults were less likely to be adherent than children (AOR for adolescents: 0.63 [95% CI: 0.52–0.75], P<0.001; AOR for adults: 0.76 [95% CI: 0.62–0.93], P=0.008). People diagnosed with ARF but not RHD were less likely to be adherent than those with severe RHD (AOR: 0.66 [95% CI: 0.53–0.81], P<0.001) as were people with mild RHD (AOR: 0.72 [95% CI: 0.59–0.89], P=0.002). People whose first diagnosis was at least 1 year ago were less likely to be adherent than those diagnosed more recently (AOR: 0.62 [95% CI: 0.52–0.75], P<0.001). People who had experienced assault were less likely to be adherent than those who had not (AOR: 0.62 [95% CI: 0.46–0.85], P=0.002), and those who had been hospitalized for hazardous alcohol use were less likely to be adherent than those who had not (AOR: 0.72 [95% CI: 0.53–0.98], P=0.035). The presence of other comorbidities had minimal effect on adherence. There was an overall increase in adherence between 2007 and 2013; 32% of people were adherent in 2013 compared with 19% in 2007 (OR: 1.58 [95% CI: 1.27–1.96], P<0.001). The proportions of people adherent in each subgroup are presented in Figures 2 and 3; see Table 3 for AORs and P values.
Figure 2.
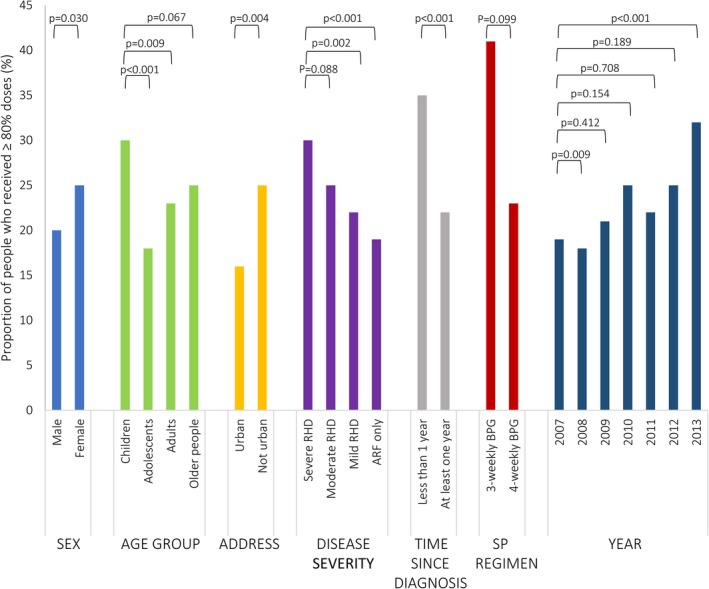
Factors associated with adherence to secondary prophylaxis (SP) for acute rheumatic fever (ARF) and rheumatic heart disease (RHD)—patient characteristics and year. P values for adjusted odds ratios from logistic regression. BPG indicates benzathine penicillin G.
Figure 3.
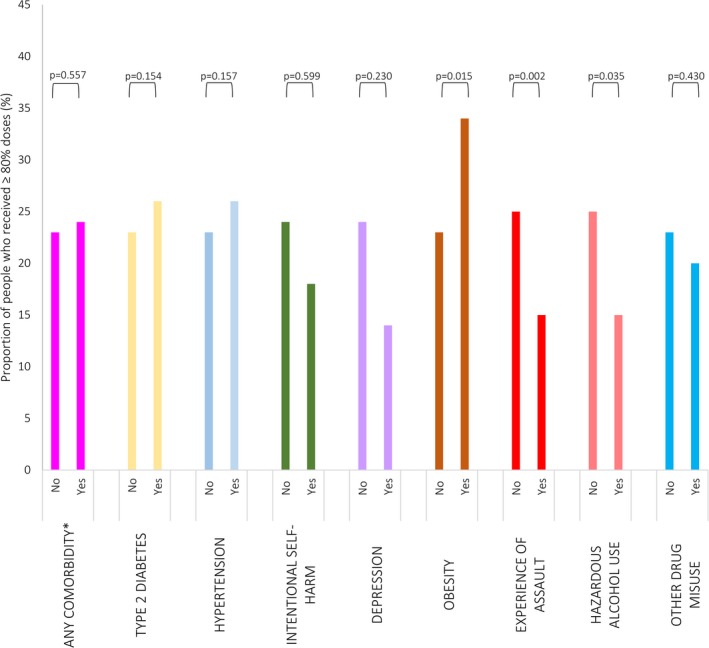
Comorbidities associated with adherence to secondary prophylaxis for acute rheumatic fever and rheumatic heart disease. *Any comorbidity included in the Charlson Comorbidity Index, P values for adjusted odds ratios from logistic regression.
Table 3.
Logistic Regression Analysis of Factors Associated With Adherence to Secondary Prophylaxis
| Observations | Adherent % (n) | Adjusted OR* of Being Adherent Compared With Reference Group (95% CI) Adherent ≥80% Doses | P Value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 2895 | 20 (584) | Ref | ··· |
| Female | 4833 | 25 (1214) | 1.21 (1.02–1.44) | 0.030† |
| Age group | ||||
| Children | 1336 | 30 (394) | Ref | |
| Adolescents | 2008 | 18 (365) | 0.63 (0.52–0.75) | <0.001† |
| Adults | 3444 | 23 (801) | 0.76 (0.62–0.93) | 0.008† |
| Older people | 940 | 25 (238) | 0.75 (0.55–1.01) | 0.054 |
| Residential address | ||||
| Urban | 1151 | 16 (188) | Ref | |
| Not urban | 6577 | 25 (1610) | 1.46 (1.13–1.88) | 0.004† |
| Disease severity | ||||
| Severe RHD | 1805 | 30 (534) | Ref | |
| Moderate RHD | 1391 | 25 (342) | 0.84 (0.69–1.02) | 0.085 |
| Mild RHD | 1804 | 22 (395) | 0.72 (0.59–0.89) | 0.002† |
| ARF only | 2728 | 19 (527) | 0.66 (0.53–0.81) | <0.001† |
| Time since first diagnosis | ||||
| Less than 1 y | 581 | 35 (203) | Ref | |
| At least 1 y | 7147 | 22 (1595) | 0.62 (0.52–0.75) | <0.001† |
| SP regimen | ||||
| 3‐wkly BPG | 66 | 41 (27) | Ref | |
| 4‐wkly BPG | 7662 | 23 (1771) | 0.53 (0.24–1.13) | 0.100 |
| Year | ||||
| 2007 | 1033 | 19 (199) | Ref | |
| 2008 | 1053 | 18 (187) | 0.74 (0.59–0.93) | 0.009† |
| 2009 | 1078 | 21 (225) | 0.91 (0.73–1.14) | 0.411 |
| 2010 | 1096 | 25 (278) | 1.17 (0.94–1.46) | 0.153 |
| 2011 | 1120 | 22 (245) | 0.96 (0.77–1.2) | 0.713 |
| 2012 | 1147 | 25 (285) | 1.16 (0.93–1.44) | 0.185 |
| 2013 | 1201 | 32 (379) | 1.58 (1.27–1.96) | <0.001† |
| Comorbidities | ||||
| Any comorbidity in CCI | ||||
| No | 5585 | 23 (1279) | Ref | |
| Yes | 2143 | 24 (519) | 1.05 (0.86–1.29) | 0.617 |
| Type 2 diabetes mellitus | ||||
| No | 6908 | 23 (1589) | ||
| Yes | 820 | 26 (209) | 1.06 (0.78–1.44) | 0.723 |
| Hypertension | ||||
| No | 6856 | 23 (1572) | Ref | |
| Yes | 872 | 26 (226) | 1.19 (0.87–1.62) | 0.281 |
| Intentional self‐harm | ||||
| No | 7412 | 24 (1742) | Ref | |
| Yes | 316 | 18 (56) | 1.15 (0.69–1.89) | 0.593 |
| Depression | ||||
| No | 7533 | 24 (1770) | Ref | |
| Yes | 195 | 14 (28) | 0.66 (0.34–1.28) | 0.217 |
| Obesity | ||||
| No | 7469 | 23 (1710) | Ref | |
| Yes | 259 | 34 (88) | 1.71 (1.11–2.63) | 0.015† |
| Experience of assault | ||||
| No | 6544 | 25 (1626) | Ref | |
| Yes | 1184 | 15 (172) | 0.62 (0.46–0.85) | 0.002† |
| Hazardous alcohol use | ||||
| No | 6456 | 25 (1602) | Ref | |
| Yes | 1272 | 15 (196) | 0.72 (0.53–0.98) | 0.035† |
| Other drug misuse | ||||
| No | 7392 | 23 (1732) | Ref | |
| Yes | 336 | 20 (66) | 1.21 (0.76–1.91) | 0.427 |
| Total | 7728 | 23 (1798) | ||
BPG indicates benzathine penicillin G; CCI, Charlson Comorbidity Index; OR, odds ratio; RHD, rheumatic heart disease; SP, secondary prophylaxis.
Adjusted for all variables listed in table.
Statistically significant difference between groups (P<0.05).
Case–Control Analysis of Adherence and Clinical Outcomes
ARF recurrences
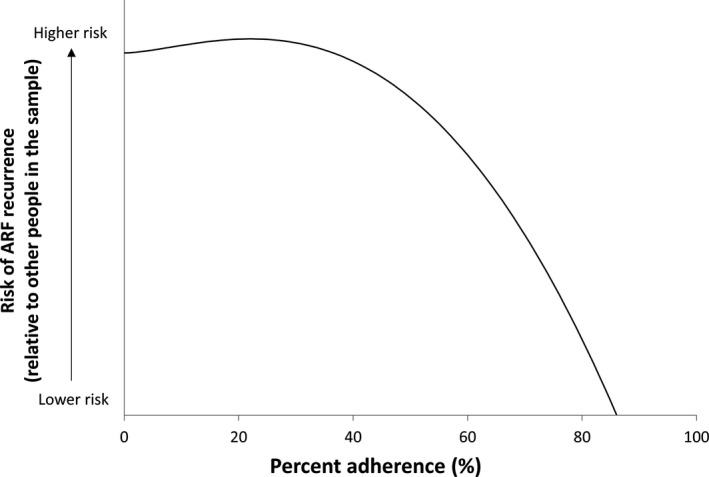
Ninety‐seven ARF recurrences were included in the case–control study, 54% of cases were female, and there was a similar proportion of cases in the child, adolescent, and adult age groups (32%, 36%, and 31%, respectively). Median adherence for cases was 46%, with 8% receiving ≥80% of doses in the 12 months before their recurrence. Median adherence for controls was 61%, with 24% receiving ≥80% of doses in the equivalent 12‐month period. The best‐fitting model was a fractional polynomial (degree 2, powers [3,3]) and the polynomial plot (Figure 4) suggests the risk of ARF recurrence did not decrease until ≈40% of doses were administered, after which increasing adherence was accompanied by a growing reduction in risk. However, the difference between the best‐fitting fractional polynomial model and the simple linear model was not statistically significant (P=0.604). The univariate linear regression model showed increasing percent adherence by 10 percentage points was associated with a 17% decrease in the odds of having a recurrence (OR: 0.83 [95% CI: 0.75–0.92], P<0.001). The AUC was 0.63 (95% CI: 0.56–0.69) (Figure 5).
Figure 4.
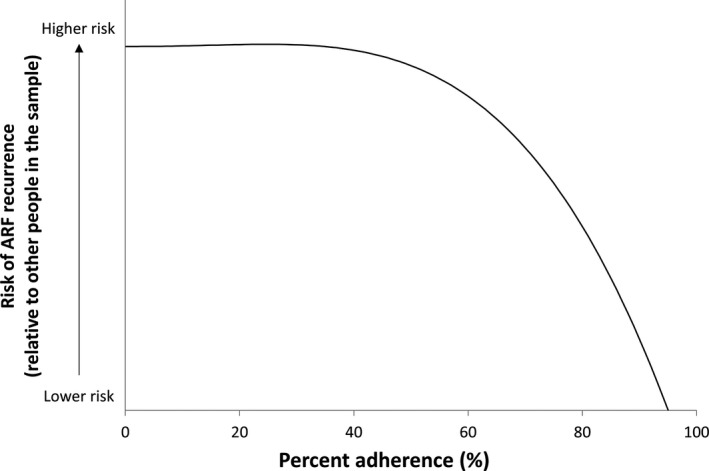
Modeled association between percent adherence and risk of acute rheumatic fever (ARF) recurrence (case–control analysis).
Figure 5.
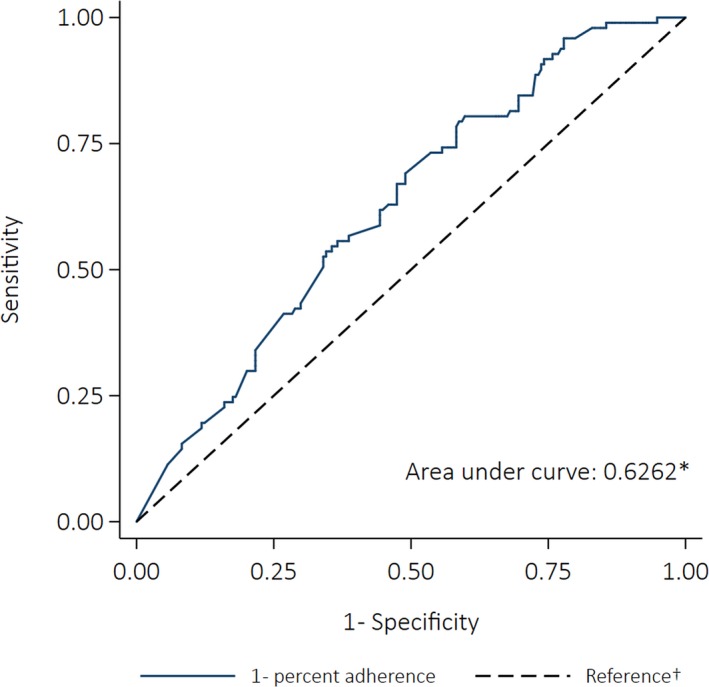
Receiver operating characteristics curve depicting the ability of percent adherence to predict acute rheumatic fever recurrence (case–control analysis). *The area under the curve was calculated using nonparametric methods; †The reference line indicates the point at which adherence would be no better at predicting an outcome than a coin toss (the area under the reference line is 0.5).
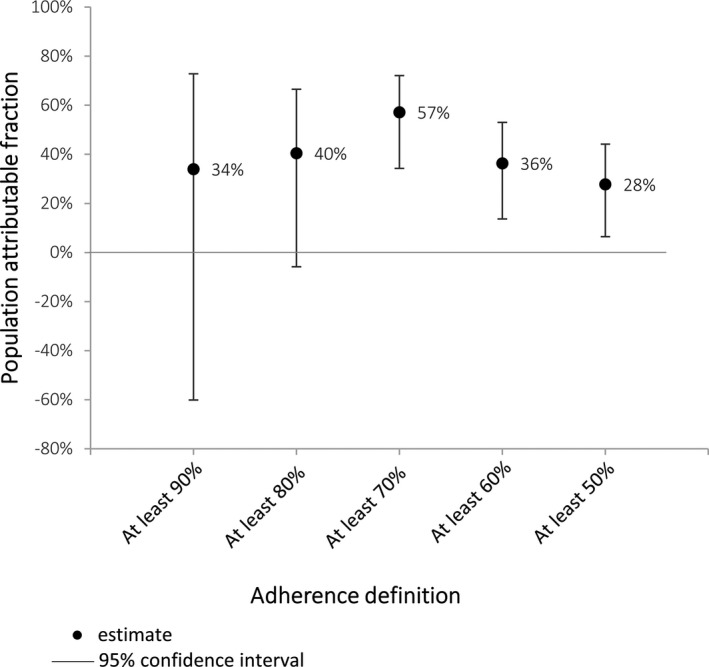
Receiving <80% of recommended doses was associated with a 4‐fold increase in the odds of having a recurrence (OR: 4.00 [95% CI: 1.72–9.29]; P=0.001). The sensitivity analysis showed significantly increased odds of ARF recurrence for all definitions of nonadherence except <30% (Table 4). The PAF implied that if everyone had received ≥80% of their doses, 69% of ARF recurrences could have been prevented (Figure 6).
Table 4.
Conditional Logistic Regression Analysis of the Association Between Different Levels of Adherence and the Risk of ARF Recurrence (Case–Control)
| Definition of Nonadherence | Case–Control Analysis 97 Cases*, 194 Controls | ||
|---|---|---|---|
| OR of Nonadherent People Having an ARF Recurrence Compared With Adherent People (95% CI) | P Value | “Adherent” Cases (n) | |
| Percent adherence <90% | 12.9 (1.68–99.11) | 0.014† | 1 |
| Percent adherence <80% | 4.00 (1.72–9.29) | 0.001† | 8 |
| Percent adherence <70% | 2.42 (1.29–4.52) | 0.006† | 19 |
| Percent adherence <60% | 2.76 (1.55–4.92) | 0.001† | 30 |
| Percent adherence <50% | 2.35 (1.36–4.07) | 0.002† | 42 |
| Percent adherence <40% | 1.87 (1.10–3.18) | 0.020† | 57 |
| Percent adherence <30% | 1.61 (0.84–3.07) | 0.149 | 73 |
ARF indicates acute rheumatic fever; OR, odds ratio.
Cases were individuals diagnosed with recurrent ARF.
Statistically significant association (P<0.05).
Figure 6.
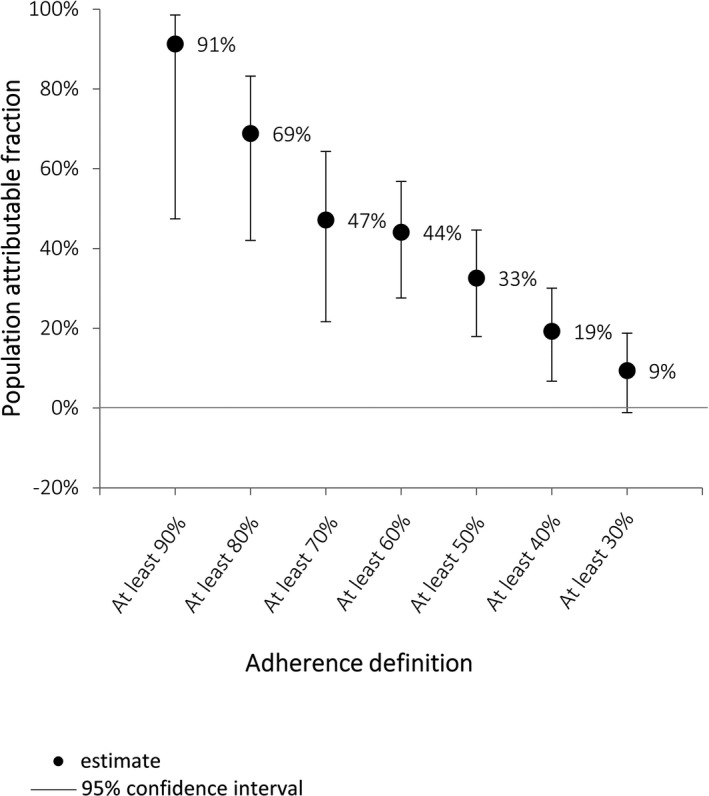
Proportion of acute rheumatic fever recurrences that could have been prevented if all people reached specified adherence definition (case–control analysis).
Progression from ARF to RHD
Sixteen people were diagnosed with RHD after a first diagnosis of ARF only. Half were males; median age at RHD diagnosis was 11.5 years (range: 6–42 years). Two of the initial ARF episodes were categorized at the time as probable or possible ARF; the remainder were recognized initially as definite cases. The time between ARF and RHD diagnosis ranged from 15 to 77 months. Seven people had a documented ARF recurrence before or at the time of RHD diagnosis (characteristics of each case are available in Table S1). Four cases could only be matched to 1 control each; all others were matched to cases as outlined in Methods.
Median percent adherence was 60% for cases (those who did develop RHD despite prescription of SP), compared with 66% for controls (those who had SP prescribed and did not develop RHD). Fractional polynomial models did not fit the data significantly better than the simple linear model (P>0.05 for all comparisons). Logistic regression showed that increasing percent adherence by 10 percentage points was associated with a statistically nonsignificant decrease in the odds of progressing to RHD (OR: 0.79 [95% CI: 0.53–1.17], P=0.238). The AUC was 0.60 (95% CI: 0.41–0.79) (Figure 7). The sensitivity analysis showed that being nonadherent was not significantly associated with the odds of progressing to RHD, though receiving fewer than 50% of doses was associated with a 4‐fold increase in risk of progressing (OR: 4.23 [95% CI: 0.46–39.14], P=0.204). Table 5 shows the odds ratios of progressing to RHD for nonadherent people compared with adherent people.
Figure 7.
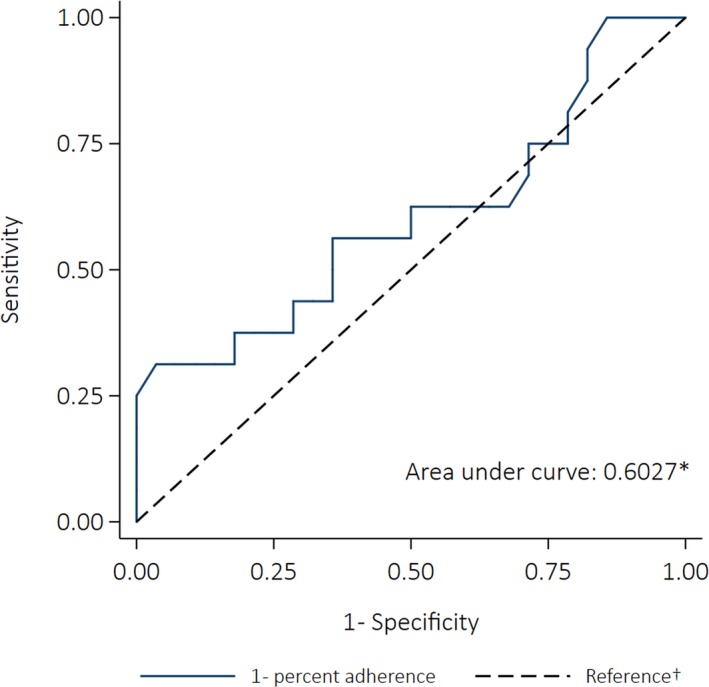
Receiver operating characteristics curve depicting the ability of percent adherence to predict progression from acute rheumatic fever to rheumatic heart disease (case–control analysis). *The area under the curve was calculated using nonparametric methods; †The reference line indicates the point at which adherence would be no better at predicting an outcome than a coin toss (the area under the reference line is 0.5).
Table 5.
Conditional Logistic Regression Analysis of Association Between Different Levels of Adherence and Progression to RHD From No Baseline RHD
| Definition of Nonadherence | Case–Control Analysis 16 Cases*, 28 Controls | ||
|---|---|---|---|
| OR of Nonadherent People Progressing to RHD Compared With Adherent People (95% CI) | P Value | “Adherent” Cases (n) | |
| Percent adherence <90% | n/a | ··· | 0 |
| Percent adherence <80% | 1.16 (0.31–4.31) | 0.825 | 4 |
| Percent adherence <70% | 1.22 (0.35–4.31) | 0.753 | 6 |
| Percent adherence <60% | 1.24 (0.34–4.48) | 0.742 | 9 |
| Percent adherence <50% | 4.23 (0.46–39.14) | 0.204 | 11 |
n/a indicates not applicable; OR, odds ratio; RHD, rheumatic heart disease.
Cases were people who progressed from acute rheumatic fever to RHD.
Progression from mild RHD to severe RHD
Six individuals progressed from mild RHD to severe RHD while prescribed SP; 4 of these people had a documented recurrence of ARF in the intervening period, and 2 did not. The small sample size meant regression analyses were not appropriate. Time to severe RHD ranged from 4 to 54 months. Adherence between the most recent mild RHD classification and first severe RHD classification ranged from 53% to 100%. Five of the 6 people received <80% of their BPG doses. Characteristics of each individual are presented in Table 6.
Table 6.
Characteristics of People Prescribed SP Who Progressed From Mild RHD to Severe RHD
| Patient Number | Sex | Age at First Severe Classification (Y) | Time Between Mild and Severe RHD (Mo) | ARF Recurrence Between Mild and Severe RHD? | Percent Adherence (%) |
|---|---|---|---|---|---|
| 1 | Female | 16 | 47 | Yes | 56 |
| 2 | Female | 25 | 54 | No | 53 |
| 3 | Male | 19 | 44 | Yes | 55 |
| 4 | Female | 15 | 36 | Yes | 69 |
| 5 | Male | 11 | 45 | Yes | 73 |
| 6 | Male | 13 | 4 | No | 100 |
ARF indicates acute rheumatic fever; RHD, rheumatic heart disease; SP, secondary prophylaxis.
Improvement from severe RHD to mild RHD
Five people improved from severe RHD to mild RHD according to available echocardiogram reports and cardiologist reviews; again, the small sample size precluded regression analyses. Time to RHD improvement ranged from 19 to 51 months; adherence ranged from 56% to 85%. Three of the 5 people received ≥80% of their BPG doses. Table 7 reports individual characteristics.
Table 7.
Characteristics of People Who Improved From Severe RHD to Mild RHD
| Patient Number | Sex | Age at First Mild RHD Classification (Y) | Time Between Severe and Mild RHD (Mo) | Percent Adherence (%) |
|---|---|---|---|---|
| 7 | Female | 5 | 19 | 56 |
| 8 | Female | 38 | 27 | 66 |
| 9 | Male | 23 | 22 | 81 |
| 10 | Female | 15 | 51 | 83 |
| 11 | Female | 12 | 22 | 85 |
RHD indicates rheumatic heart disease.
All‐cause mortality
Sixty‐nine deaths were included; 64% of the cases were female and 66% were <41 years old at the time of death. Eighty‐four percent had severe RHD at the time of death. Median adherence for cases was 38%, compared with 60% for controls. Fractional polynomial models did not fit the relationship between adherence and death better than the simple linear model (P=0.061 for comparison of best‐fitting fractional polynomial model with linear model). Univariate linear regression showed increasing percent adherence by 10 percentage points was associated with a 12% decrease in the odds of dying (OR: 0.88 [95% CI: 0.80–0.97], P=0.009). The AUC was 0.60 (95% CI: 0.53–0.68) (Figure 8). The sensitivity analysis indicated that receiving <80% of doses almost doubled the odds of death (OR: 1.90 [95% CI: 0.89–4.06], P=0.099), though the association was not statistically significant. However, 3 other definitions of nonadherence (<70%, <60%, and <50%) were significantly associated with an increased likelihood of death (Table 8). The PAFs implied that 57% (95% CI: 34% to 72%) of deaths could have been prevented if everyone had received ≥70% of doses (Figure 9). The reductions in deaths that could have resulted from everyone attaining ≥80% and ≥90% were not significant (40% [95% CI: −6% to 67%] and 34% [95% CI: −60% to 73%], respectively).
Figure 8.
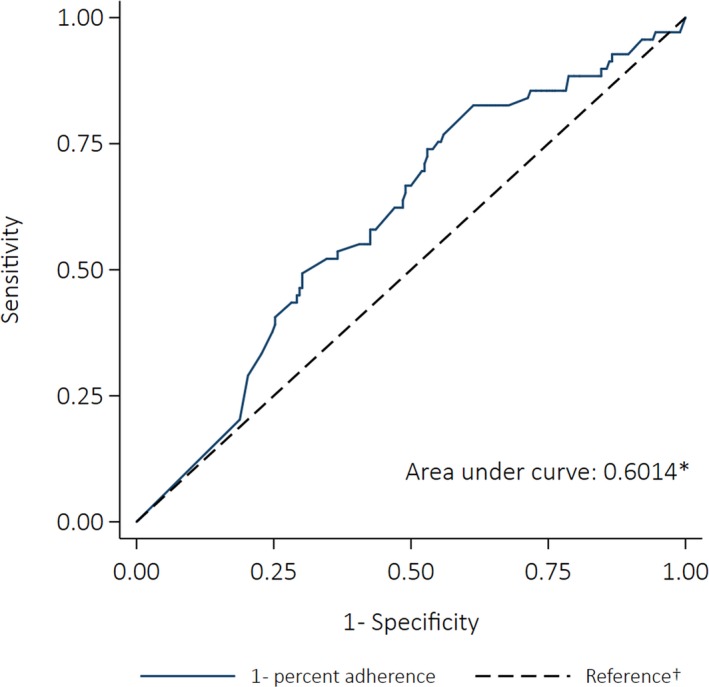
Receiver operating characteristics curve for the ability of percent adherence to predict all‐cause mortality (case–control analysis). *The area under the curve was calculated using nonparametric methods; †The reference line indicates the point at which adherence would be no better at predicting an outcome than a coin toss (the area under the reference line is 0.5).
Table 8.
Logistic Regression Analysis of the Association Between Different Levels of Adherence and the Odds of All‐Cause Mortality
| Definition of Nonadherence | Case–Control Analysis 69 Cases*, 202 Controls | ||
|---|---|---|---|
| OR of Nonadherent People Dying Compared With Adherent People (95% CI) | P Value | “Adherent” Cases (n) | |
| Percent adherence <90% | 1.58 (0.58–4.27) | 0.369 | 5 |
| Percent adherence <80% | 1.90 (0.89–4.06) | 0.099 | 10 |
| Percent adherence <70% | 3.25 (1.58–6.68) | 0.001† | 12 |
| Percent adherence <60% | 2.20 (1.16–4.16) | 0.016† | 23 |
| Percent adherence <50% | 1.92 (1.03–3.56) | 0.039† | 29 |
OR indicates odds ratio.
Cases were people who died.
Statistically significant result (P<0.05).
Figure 9.
Proportion of deaths that could have been prevented if all people reached specified adherence definition (case–control analysis).
Case–Crossover Analysis
ARF recurrences
Fifty‐eight people could be included in the case–crossover analysis; 50% were female. The proportion of cases who were children was smaller compared with the case–control cases (21%); 41% were adolescents, 36% were adults, and 1 person was >40 years. Median adherence in the first control window (when no ARF recurrence occurred) was 51% (10 people received ≥80% of doses). Median adherence fell to 46% in the 12 months before the recurrence (the case window) (5 people received ≥80% of doses). Adherence increased to 69% in the 12‐month period after the recurrence (control window 2) (15 people received ≥80% doses). The best‐fitting model was a fractional polynomial (degree 2, powers [2,2]). The fractional polynomial plot (Figure 10) indicates the risk of ARF recurrence decreased once ≈25% of doses were administered, and the risk continued to reduce with increasing adherence. The difference between the best‐fitting fractional polynomial model and the simple linear model was not statistically significant (P=0.220). Increasing percent adherence by 10 percentage points was associated with a 21% decrease in the odds of having a recurrence (OR: 0.79 [95% CI: 0.67–0.94], P=0.006). The AUC was 0.61 (95% CI: 0.53–0.70) (Figure 11).
Figure 10.
Modeled association between percent adherence and risk of acute rheumatic fever (ARF) recurrence (case–crossover analysis).
Figure 11.
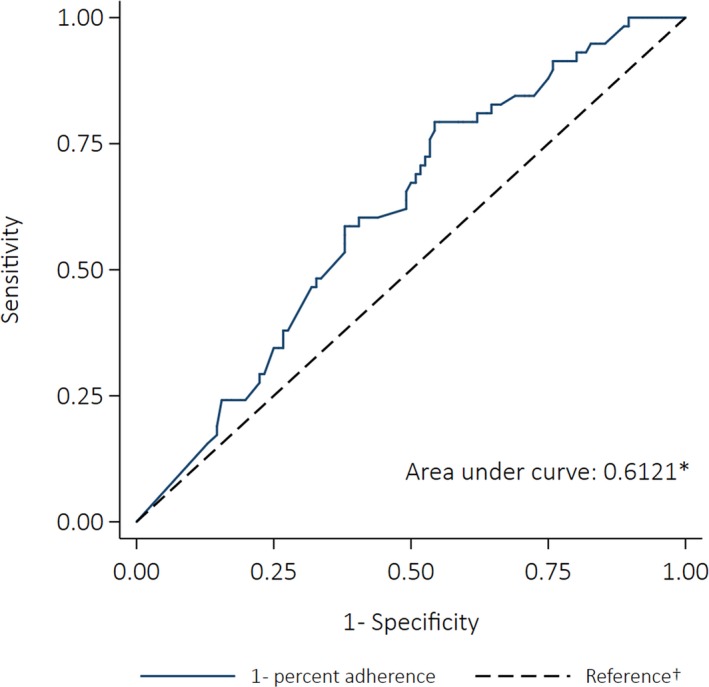
Receiver operating characteristics curve depicting the ability of percent adherence to predict acute rheumatic fever recurrence (case–crossover analysis). *The area under the curve was calculated using nonparametric methods; †The reference line indicates the point at which adherence would be no better at predicting an outcome than a coin toss (the area under the reference line is 0.5).
The logistic regression analyses showed all definitions of nonadherence, except <30%, were associated with significantly increased risk of ARF recurrence (Table 9). People who received <80% of doses were ≈3 times more likely to have a recurrence than those who received ≥80% (OR: 3.31 [95% CI: 1.09–10.07]; P=0.035). Sixty‐four percent of ARF recurrences could have been prevented if everyone had received ≥80% doses (Figure 12).
Table 9.
Logistic Regression Analysis of the Association Between Different Levels of Adherence and the Risk of ARF Recurrence (Case–Crossover Analysis, 58 Cases)
| Definition of Nonadherence | OR of Nonadherent People Having an ARF Recurrence Compared With Adherent People (95% CI) | P Value | “Adherent” People in Case Window (n) |
|---|---|---|---|
| Percent adherence <90% | n/a | n/a | 0 |
| Percent adherence <80% | 3.31 (1.09–10.07) | 0.035* | 5 |
| Percent adherence <70% | 5.02 (1.68–15.03) | 0.004* | 12 |
| Percent adherence <60% | 3.89 (1.44–10.49) | 0.007* | 16 |
| Percent adherence <50% | 2.68 (1.16–6.20) | 0.021* | 23 |
| Percent adherence <40% | 2.38 (1.04–5.43) | 0.040* | 31 |
| Percent adherence <30% | 1.59 (0.66–3.85) | 0.305 | 41 |
ARF indicates acute rheumatic fever; n/a, not applicable; OR, odds ratio.
Statistically significant result (P<0.05).
Figure 12.
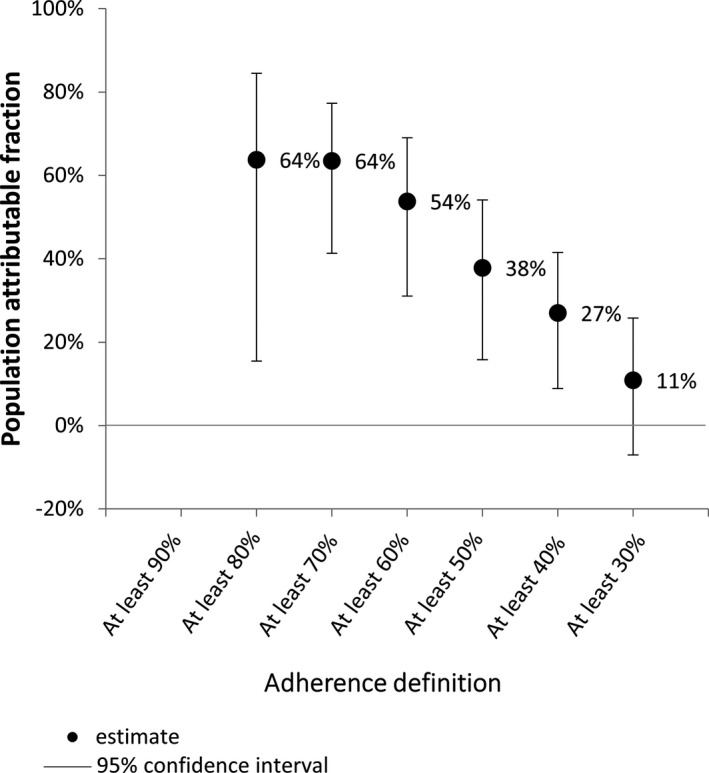
Proportion of acute rheumatic fever recurrences that could have been prevented if all people reached specified adherence definition (case–crossover analysis).
Progression from mild RHD to severe RHD, and improvement from severe RHD to mild RHD
The small sample sizes available precluded any statistical analyses.
All‐cause mortality
Forty‐three deaths were included; 58% were female and 48% were >40 years old at the time of death. Eighty‐eight percent had severe RHD when they died. Median adherence in the control windows was 46% and 49%, and dropped to 38% in the 12 months before death. Fractional polynomial models did not fit the relationship between adherence and death better than the simple linear model (P=0.428 for the comparison of the best‐fitting fractional polynomial model and the linear model). The results of the univariate linear regression showed that increasing percent adherence by 10% points was associated with a 9% decrease in the odds of dying (OR: 0.91 [95% CI: 0.74–1.11], P=0.348).
The AUC was 0.53 (95% CI: 0.42–0.63) (Figure 13). The sensitivity analyses showed no significant associations between any of the definitions of nonadherence and the odds of dying (Table 10). However, each of the ORs was >1, suggesting that people who were not adherent had a greater likelihood of dying. The PAFs indicated that deaths could have been prevented if everyone had been adherent, regardless of the definition of adherence; however, none of the PAFs were statistically significant (Figure 14).
Figure 13.
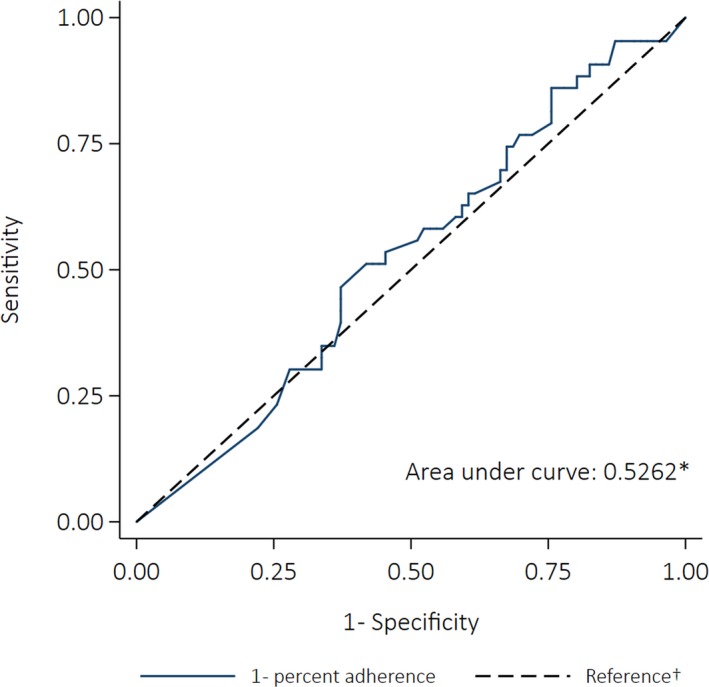
Receiver operating characteristics curve for the ability of percent adherence to predict all‐cause mortality (case–crossover analysis). *The area under the curve was calculated using nonparametric methods. †The reference line indicates the point at which adherence would be no better at predicting an outcome than a coin toss (the area under the reference line is 0.5).
Table 10.
Logistic Regression Analysis of the Association Between Different Levels of Adherence and the Odds of All‐Cause Mortality (Case–Crossover Analysis, 43 Cases)
| Definition of Nonadherence | OR of Nonadherent People Dying Compared With Adherent People (95% CI) | P Value | “Adherent” People in Case Window (n) |
|---|---|---|---|
| Percent adherence <90% | 5.16 (0.58–45.87) | 0.141 | 4 |
| Percent adherence <80% | 1.91 (0.51–7.12) | 0.334 | 8 |
| Percent adherence <70% | 2.00 (0.56–7.09) | 0.170 | 10 |
| Percent adherence <60% | 1.33 (0.46–3.87) | 0.394 | 15 |
| Percent adherence <50% | 1.19 (0.43–3.27) | 0.732 | 18 |
OR indicates odds ratio.
Figure 14.
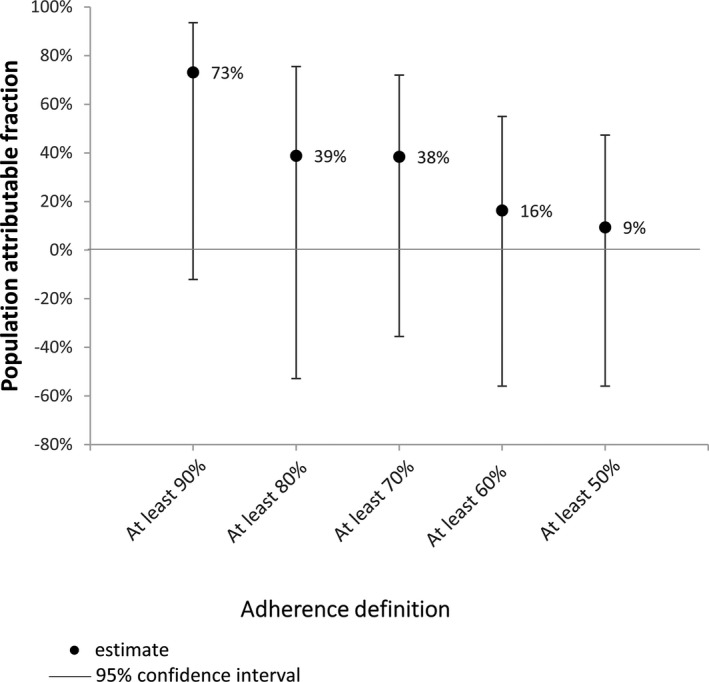
Proportion of deaths that could have been prevented if all people reached specified adherence definition (case–crossover analysis).
Discussion
This is the first comprehensive analysis of the effectiveness of SP at a population level. We confirm that SP is a critical tool for RHD control, with increased adherence leading to reduced risk of ARF recurrence and death. Furthermore, our data provide tangible guidance for those managing health systems as well as for clinicians and clients managing RHD control at the individual level. At the system level, our findings support the programmatic use of ≥80% as an adherence target, since greatest benefit was achieved at this level of adherence. It appeared that higher levels of protection occurred at ≥90% adherence, and this is a logical assumption, but too few individuals were in this category for robust assessment, and the ≥80% target (≥11 out of 13 annual injections for people prescribed 4‐weekly BPG) is programmatically more feasible in our setting.
At the individual level, we demonstrated a threshold effect at 40% of injections delivered. If this is confirmed, it provides a rationale for a particular focus on those with the lowest levels of adherence to bring them over the 40% threshold. Above this level, the message is clear: every dose counts. Indeed, for every 10% increase in adherence, the odds of ARF recurrence reduced by 17%. This is a powerful motivational tool for clients. These data can be used by healthcare providers to explain to their patients the likely benefit they will receive at different levels of adherence.
We included case–crossover analyses as well as a case–control design in order to reduce biases that could occur in comparing different individuals who may have different characteristics including host immune responses and exposure to GAS. The similarity of the case–control and case–crossover results supports the validity of the findings. Numbers in the case–crossover study were smaller, and less likely to show statistical significance, but the associations at all definitions of adherence indicated that increased adherence was beneficial.
This study identifies important patient characteristics associated with adherence to SP for ARF and RHD. Increased adherence in females and people with more severe RHD has been previously reported in this setting,23 and older age and increasing time since diagnosis has been associated with reduced adherence in other populations.24, 25, 26 The results indicate that obesity was associated with higher adherence. This may be a spurious finding (eg, because of bias in documentation of obesity) or may reflect a genuine relationship. Documentation of obesity may be a marker of greater healthcare engagement, since documentation of obesity in the medical records may be overlooked for people who present rarely and who have more apparently urgent health priorities, whereas healthcare providers have greater time to recognize and document low‐acuity conditions such as obesity for regular attenders. A real association may exist if people with obesity are genuinely more engaged in health care because of comorbidities such as diabetes mellitus requiring more regular attendance (although the relationship with diabetes mellitus was not statistically significant). Also, some healthcare providers in the NT have suggested that people with low body mass index experience greater pain from BPG injections, and the converse may apply to people with higher body mass indexes, ie: they experience less pain, and therefore may be more likely to receive BPG injections. However, this suggestion has not been tested in our setting (or elsewhere). The findings also indicate that hazardous alcohol use and experience of assault were associated with lower adherence. Many Australian Aboriginal people are negatively affected by alcohol use (their own or another person's), and exposure to violence.27 Hazardous alcohol use and experience of assault suggest unstable living circumstances, which could impede a person's ability to adhere to medication regimens, which may explain these results.
The higher rates of adherence found in children may be because of them receiving more family support than adolescents and adults; this may also be an indication that healthcare providers have prioritized adherence support for children since they are at greatest risk of GAS infection. In contrast to our study, a systematic review reported that living in urban areas was associated with higher adherence.5 Many Indigenous Australians in the NT live in remote communities that are serviced by a single local health clinic; access to SP in urban settings may be more difficult since there are multiple health services spread over a much larger geographic area. People with lower adherence should be provided additional support; proven, culturally sensitive, tailored strategies should be implemented. Recommendations for enhanced support of Aboriginal Australians with chronic conditions28 and for Aboriginal adolescents with a history of ARF or RHD have recently been published.29 Adherence is the responsibility of health systems working in partnership with patients. Work to enhance health systems and improve treatment options must continue.
While overall adherence levels were disappointing, improvements in adherence over time are a testament to the resilience of patients and their families, and the support of primary health services and the NT RHD Control Program.
Being “adherent” or “compliant” has been associated with reduced ARF recurrences in other settings6, 7, 8, 9, 10, 11, 12, 13, 14; however, definitions of adherence were not always reported and continuous measures of adherence rarely analyzed, so the required level of adherence to achieve optimal clinical benefit has remained unclear, as has the minimum level of adherence required to obtain any benefit. The only other analysis of percent adherence as a continuous measure reported that adherence was not significantly associated with reduced risk of recurrent ARF.30 The method of calculating adherence for that study was not reported in detail, so it is difficult to compare the results with our findings.
In our study, some people were diagnosed with an ARF recurrence or progressed to RHD despite receiving ≥80% of doses. Percent adherence does not account for the timing of doses, and when doses are administered more than 28 days apart (for the 4‐weekly regimen) there can be opportunities for GAS infection and consequent development of recurrent ARF, which may explain adverse outcomes despite high percent adherence. So‐called “breakthrough” recurrences have been reported for populations outside Australia,14, 31, 32 and may also be explained by faster metabolism of penicillin by some individuals.33 Research is under way to assess the pharmacokinetics and pharmacodynamics of benzathine penicillin in the study population. Despite children having higher rates of adherence than the older age groups, the rate of ARF recurrences was similar for each age group. This may be because of the increased vulnerability of children to GAS infections, which means higher rates of adherence are required to prevent recurrence.
It is thought that after the resolution of an initial episode of ARF, further damage to the heart valves is only brought about by recurrent ARF. In our study, 9 cases progressed from ARF to RHD without a documented intervening episode of ARF; it is possible that there was an intervening undiagnosed or subclinical recurrence of ARF. There was no clear trend between adherence and progression to RHD, and 3 people progressed despite receiving ≥80% of doses. Patients often first present to health services with established RHD both in Australia and internationally.34 In this patient data set, 1213/2829 (43%) had a diagnosis of RHD with no prior ARF diagnosis. Therefore, there are limited international data on the relationship between adherence and progression from ARF to RHD. The only published study identified reported on a cohort of 6 people with ARF; 4 people progressed to RHD during the 4 years of follow‐up: 2 had received at least 75% of doses, and 2 were not adherent.35
In this cohort, 3 of 5 individuals who improved from severe RHD to mild RHD achieved ≥80% adherence, but only 1 of 6 whose RHD significantly worsened achieved high adherence, and this case—individual number 6 (Table 6)—appeared to have had evolving carditis from a protracted severe episode of ARF, and antibiotic adherence in this context was unlikely to have been able to affect their outcome.
Before the introduction of antibiotic prophylaxis, it was observed that heart valve lesions may resolve in ≈10% of patients,36 but prophylaxis has been shown to dramatically improve prognosis, and Tompkins et al reported in 1972 that acute mitral regurgitation resolved in 70% of patients with ARF who were adherent to 4‐weekly BPG for at least 5 years.37 These early studies used auscultation to determine disease severity rather than echocardiography,38 so direct comparison with our study findings is not possible. A more recent study measured changes in valve lesion severity and all patients prescribed SP (n=6) had regression of mild lesions, but the methods of measuring valvular structure and function and calculating adherence were not described. A fourth study used valvular regurgitation as an indication of disease severity; regurgitation improved in 7 of the 13 people who received ≥75% of doses and did not improve in any of the people who were not adherent (n=4).35 Our findings add to the existing body of literature by illustrating that even severe RHD can markedly improve, but this is uncommon.
Deaths in this cohort occurred at a young age—almost two thirds were aged <40 years at the time of death. Eighty‐eight percent of those dying had severe RHD compared with 23% in the overall cohort. It is likely that RHD was a contributing factor in a number of instances, but data on cause of death were unavailable. Our study found that penicillin adherence appeared to be protective against all‐cause mortality, although the sensitivity analysis showed the highest levels of adherence were not more protective than lower levels of adherence. Given the specific action of BPG, and high prevalence of comorbidities in this population, a clear linear association was not expected. In the better‐powered case–control analysis, increasing adherence was protective against all‐cause mortality, with a 12% lower risk of dying for each 10 percentage point increase in adherence. A large multinational study has reported no significant association between SP and all‐cause mortality.39 One other study reported that increased adherence to SP reduced the risk of RHD‐related death34; while this is feasible, the time frame for adherence calculations was <3 months for many participants, so it is possible that adherence to SP was a proxy for adherence to other medications that contributed to reduced mortality.
Key strengths of this study are the large cohort, the use of data on SP recorded by health providers, and the use of 2 complementary study designs that provided an indication of validity. ARF is notifiable in the NT, so ARF diagnoses are well documented relative to other settings. Study limitations include the small numbers of cases for some outcomes that prevented rigorous statistical analyses; data from larger cohorts might clarify the role of SP in the prevention or amelioration of RHD. Adherence to medication is known to be influenced by a range of factors, and we did not have access to data on all potentially relevant factors, such as socioeconomic status, prescription of concomitant medications, or mode of delivery of health care (eg, clinic‐based or outreach service). However, another study recently conducted in this setting examined relationships between adherence and 17 clinic and community characteristics, including outreach service delivery, and found no significant associations.40 Another limitation is the subjectivity of the interpretation of RHD severity using echocardiograms, which we addressed in part by only including large changes in severity; although imperfect, the use of echocardiograms is a substantial improvement on auscultation, which has been used in historical studies. The study period may not have been sufficiently long to capture change in disease severity or death. Finally, only 1 measure of adherence was analyzed; alternative measures of adherence are being explored as part of this program and may strengthen our understanding of the association between adherence and clinical outcomes.
Conclusion
We show for the first time that increased adherence to SP is associated with a reduction in ARF recurrence in our setting. An association with improved clinical outcome was observed across all levels of increasing adherence, but for practical purposes at programmatic level and for healthcare providers, monitoring dichotomized measures of adherence may be the most feasible approach, particularly where the quality of adherence data is uncertain. RHD Control Programs could continue using a target of ≥80%, but further examination of “breakthrough” recurrences is required. For those already diagnosed with ARF or RHD, SP up to the recommended age limit is crucial, but the majority of cases already have RHD at baseline, limiting the potential benefit of SP. The social determinants of health must therefore be addressed alongside innovative approaches including GAS vaccine development and ongoing initiatives to support adherence, in order to reduce the burden of this disease.
Sources of Funding
de Dassel is supported by an Australian Postgraduate Award Scholarship. Ralph has been supported by the Australian National Health and Medical Research Council (NHMRC) fellowships 1142011 and 1113638 and the END RHD Centre of Research Excellence (NHMRC 1080401).
Disclosures
None.
Supporting information
Table S1. Characteristics of People Who Progressed From Acute Rheumatic Fever to Rheumatic Heart Disease
(J Am Heart Assoc. 2018;7:e010223 DOI: 10.1161/JAHA.118.010223)
References
- 1. Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, Nascimento BR, Ribeiro ALP, Sable CA, Steer AC, Naghavi M, Mokdad AH, Murray CJL, Vos T, Carapetis JR, Roth GA. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017;377:713–722. [DOI] [PubMed] [Google Scholar]
- 2. Stollerman GH. The use of antibiotics for the prevention of rheumatic fever. Am J Med. 1954;17:757–767. [DOI] [PubMed] [Google Scholar]
- 3. Chamovitz R, Catanzaro FJ, Stetson CA, Rammelkamp CH Jr. Prevention of rheumatic fever by treatment of previous streptococcal infections. I. Evaluation of benzathine penicillin G. N Engl J Med. 1954;251:466–471. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Rheumatic Fever and Rheumatic Heart Disease: Report of a WHO Expert Consultation Geneva, 29 October–1 November 2001. Geneva: World Health Organization; 2004. [Google Scholar]
- 5. Kevat PM, Reeves BM, Ruben AR, Gunnarsson R. Adherence to secondary prophylaxis for acute rheumatic fever and rheumatic heart disease: a systematic review. Curr Cardiol Rev. 2017;13:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grover A, Dhawan A, Iyengar SD, Anand IS, Wahi PL, Ganguly NK. Epidemiology of rheumatic fever and rheumatic heart disease in a rural community in northern India. Bull World Health Organ. 1993;71:59–66. [PMC free article] [PubMed] [Google Scholar]
- 7. Roodpeyma S, Kamali Z, Zare R. Rheumatic fever: the relationship between clinical manifestations and laboratory tests. J Paediatr Child Health. 2005;41:97–100. [DOI] [PubMed] [Google Scholar]
- 8. Chen L, Xie X, Gu J, Xu L, Yang X, Yu B. Changes of manifestations of 122 patients with rheumatic fever in South China during last decade. Rheumatol Int. 2009;30:239–243. [DOI] [PubMed] [Google Scholar]
- 9. Spinetto H, Lennon D, Horsburgh M. Rheumatic fever recurrence prevention: a nurse‐led programme of 28‐day penicillin in an area of high endemnicity. J Paediatr Child Health. 2011;47:228–234. [DOI] [PubMed] [Google Scholar]
- 10. Bassili A, Zaher SR, Zaki A, Abdel‐Fattah M, Tognoni G. Profile of secondary prophylaxis among children with rheumatic heart disease in Alexandria, Egypt. East Mediterr Health J. 2000;6:437–446. [PubMed] [Google Scholar]
- 11. Nordet P, Lopez R, Dueñas A, Sarmiento L. Prevention and control of rheumatic fever and rheumatic heart disease: the Cuban experience (1986–1996–2002). Cardiovasc J Afr. 2008;19:135–140. [PMC free article] [PubMed] [Google Scholar]
- 12. Majeed HA, Yousof AM, Khuffash FA, Yusuf AR, Farwana S, Khan N. The natural history of acute rheumatic fever in Kuwait: a prospective six year follow‐up report. J Chronic Dis. 1986;39:361–369. [DOI] [PubMed] [Google Scholar]
- 13. Lue HC, Wu MH, Wang JK, Wu FF, Wu YN. Long‐term outcome of patients with rheumatic fever receiving benzathine penicillin G prophylaxis every three weeks versus every four weeks. J Pediatr. 1994;125:812–816. [DOI] [PubMed] [Google Scholar]
- 14. Pelajo CF, Lopez‐Benitez JM, Torres JM, de Oliveira SK. Adherence to secondary prophylaxis and disease recurrence in 536 Brazilian children with rheumatic fever. Pediatr Rheumatol Online J. 2010;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roberts KV, Maguire GP, Brown A, Atkinson DN, Remenyi B, Wheaton G, Ilton M, Carapetis J. Rheumatic heart disease in Indigenous children in northern Australia: differences in prevalence and the challenges of screening. Med J Aust. 2015;203:221.e1–7. [DOI] [PubMed] [Google Scholar]
- 16. AIHW , Field B. Rheumatic Heart Disease: All But Forgotten in Australia Except Among Aboriginal and Torres Strait Islander Peoples. Bulletin no. 16. AIHW Cat. No. AUS 48. Canberra: AIHW; 2004. [Google Scholar]
- 17. He VY, Condon JR, Ralph AP, Zhao Y, Roberts K, de Dassel JL, Currie BJ, Fittock M, Edwards KN, Carapetis JR. Long‐term outcomes from acute rheumatic fever and rheumatic heart disease: a data‐linkage and survival analysis approach. Circulation. 2016;134:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Dassel JL, Fittock MT, Wilks SC, Poole JE, Carapetis JR, Ralph AP. Adherence to secondary prophylaxis for rheumatic heart disease is underestimated by register data. PLoS One. 2017;12:e0178264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. RHDAustralia (ARF/RHD writing group), National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand . Australian Guideline for Prevention, Diagnosis and Management of Acute Rheumatic Fever and Rheumatic Heart Disease. 2nd ed Darwin, Australia: Menzies School of Health Research; 2012. [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 21. Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. [DOI] [PubMed] [Google Scholar]
- 22. Gray LA, D'Antoine HA, Tong SYC, McKinnon M, Bessarab D, Brown N, Remenyi B, Steer A, Syn G, Blackwell GM, Inouye M, Carapetis JR. Genome‐wide analysis of genetic risk factors for rheumatic heart disease in aboriginal Australians provides support for pathogenic molecular mimicry. J Infect Dis. 2017;216:1460–1470. [DOI] [PubMed] [Google Scholar]
- 23. Eissa S, Lee R, Binns P, Garstone G, McDonald M. Assessment of a register‐based rheumatic heart disease secondary prevention program in an Australian Aboriginal community. Aust N Z J Public Health. 2005;29:521–525. [DOI] [PubMed] [Google Scholar]
- 24. Quinn RW, Federspiel CF, Lefkowitz LB, Christie AU. Recurrences and sequelae of rheumatic fever in Nashville. A follow‐up study. JAMA. 1977;238:1512–1515. [PubMed] [Google Scholar]
- 25. Longenecker CT, Morris SR, Aliku TO, Beaton A, Costa MA, Kamya MR, Kityo C, Lwabi P, Mirembe G, Nampijja D, Rwebembera J, Sable C, Salata RA, Scheel A, Simon DI, Ssinabulya I, Okello E. Rheumatic heart disease treatment cascade in Uganda. Circ Cardiovasc Qual Outcomes. 2017;10:e004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zuhlke L, Engel ME, Karthikeyan G, Rangarajan S, Mackie P, Cupido B, Mauff K, Islam S, Joachim A, Daniels R, Francis V, Ogendo S, Gitura B, Mondo C, Okello E, Lwabi P, Al‐Kebsi MM, Hugo‐Hamman C, Sheta SS, Haileamlak A, Daniel W, Goshu DY, Abdissa SG, Desta AG, Shasho BA, Begna DM, ElSayed A, Ibrahim AS, Musuku J, Bode‐Thomas F, Okeahialam BN, Ige O, Sutton C, Misra R, Abul Fadl A, Kennedy N, Damasceno A, Sani M, Ogah OS, Olunuga T, Elhassan HH, Mocumbi AO, Adeoye AM, Mntla P, Ojji D, Mucumbitsi J, Teo K, Yusuf S, Mayosi BM. Characteristics, complications, and gaps in evidence‐based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J. 2015;36:1115–1122a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Australian Health Ministers’ Advisory Council . Aboriginal and Torres Strait Islander Health Performance Framework 2017 Report. Canberra: AHMAC; 2017. [Google Scholar]
- 28. de Dassel JL, Ralph AP, Cass A. A systematic review of adherence in Indigenous Australians: an opportunity to improve chronic condition management. BMC Health Serv Res. 2017;17:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mitchell AG, Belton S, Johnston V, Ralph AP. Transition to adult care for Aboriginal children with rheumatic fever: a review informed by a focussed ethnography in northern Australia. Aust J Prim Health. 2018;24:9–13. [DOI] [PubMed] [Google Scholar]
- 30. Seckeler MD, Hoke TR, Gurka MJ, Barton LL. No demonstrable effect of benzathine penicillin on recurrence of rheumatic fever in Pacific Island population. Pediatr Cardiol. 2010;31:849–852. [DOI] [PubMed] [Google Scholar]
- 31. Terreri MT, Roja SC, Len CA, Faustino PC, Roberto AM, Hilario MO. Sydenham's chorea—clinical and evolutive characteristics. Sao Paulo Med J. 2002;120:16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berrios X, Quesney F, Morales A, Blazquez J, Bisno AL. Are all recurrences of “pure” Sydenham chorea true recurrences of acute rheumatic fever? J Pediatr. 1985;107:867–872. [DOI] [PubMed] [Google Scholar]
- 33. Broderick MP, Hansen CJ, Faix DJ. Factors associated with loss of penicillin G concentrations in serum after intramuscular benzathine penicillin G injection: a meta‐analysis. Pediatr Infect Dis J. 2012;31:722–725. [DOI] [PubMed] [Google Scholar]
- 34. Okello E, Longenecker CT, Beaton A, Kamya MR, Lwabi P. Rheumatic heart disease in Uganda: predictors of morbidity and mortality one year after presentation. BMC Cardiovasc Disord. 2017;17:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haran S, Crane N, Kazi S, Axford‐Haines L, White A. Effect of secondary penicillin prophylaxis on valvular changes in patients with rheumatic heart disease in Far North Queensland. Aust J Rural Health. 2018;26:119–125. [DOI] [PubMed] [Google Scholar]
- 36. Bland EF, Duckett Jones T. Rheumatic fever and rheumatic heart disease; a twenty year report on 1000 patients followed since childhood. Circulation. 1951;4:836–843. [DOI] [PubMed] [Google Scholar]
- 37. Tompkins DG, Boxerbaum B, Liebman J. Long‐term prognosis of rheumatic fever patients receiving regular intramuscular benzathine penicillin. Circulation. 1972;45:543–551. [DOI] [PubMed] [Google Scholar]
- 38. Remenyi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, Lawrenson J, Maguire G, Marijon E, Mirabel M, Mocumbi AO, Mota C, Paar J, Saxena A, Scheel J, Stirling J, Viali S, Balekundri VI, Wheaton G, Zuhlke L, Carapetis J. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence‐based guideline. Nat Rev Cardiol. 2012;9:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zühlke L, Karthikeyan G, Engel ME, Rangarajan S, Mackie P, Cupido‐Katya Mauff B, Islam S, Daniels R, Francis V, Ogendo S, Gitura B, Mondo C, Okello E, Lwabi P, Al‐Kebsi MM, Hugo‐Hamman C, Sheta SS, Haileamlak A, Daniel W, Goshu DY, Abdissa SG, Desta AG, Shasho BA, Begna DM, ElSayed A, Ibrahim AS, Musuku J, Bode‐Thomas F, Yilgwan CC, Amusa GA, Ige O, Okeahialam B, Sutton C, Misra R, Abul Fadl A, Kennedy N, Damasceno A, Sani MU, Ogah OS, Elhassan TO, Mocumbi AO, Adeoye AM, Mntla P, Ojji D, Mucumbitsi J, Teo K, Yusuf S, Mayosi BM. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low‐ and middle‐income countries: two‐year follow‐up of the global rheumatic heart disease registry (the REMEDY study). Circulation. 2016;134:1456–1466. [DOI] [PubMed] [Google Scholar]
- 40. Ralph AP, de Dassel JL, Kirby A, Read C, Mitchell AG, Maguire GP, Currie BJ, Bailie RS, Johnston V, Carapetis JR. Improving delivery of secondary prophylaxis for rheumatic heart disease in a high‐burden setting: outcome of a stepped‐wedge, community randomized trial. J Am Heart Assoc. 2018;7:e009308 DOI: 10.1161/JAHA.118.009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of People Who Progressed From Acute Rheumatic Fever to Rheumatic Heart Disease


