The FIRE AND ICE Trial (ClinicalTrials.gov, identifier NCT01490814) was initiated in 2012 as a multicenter, randomized, head‐to‐head comparison of radiofrequency current (RFC) and cryoballoon catheter ablation for the treatment of patients with drug‐refractory symptomatic paroxysmal atrial fibrillation (AF). Six years on, it remains the largest, randomized comparison of safety and efficacy between 2 catheter ablation modalities used in the treatment of patients with AF. This landmark trial not only established noninferiority between cryoballoon and RFC ablation for pulmonary vein isolation (PVI) with regard to the study's efficacy and safety primary end points,1 but also, it evaluated secondary end points that were critical for a representative study interpretation.
In congruence with our trial data presentations, Pocock and Stone discussed characteristics of a clinical trial that should be evaluated to ascertain clinically meaningful outcomes from statistically positive trials.2 Specifically, their review focused on the balanced interpretation of clinical evidence. In addition to the magnitude of benefit, the size of the trial, the balance between safety and efficacy, and the specificity of the results to a select patient population, the authors discuss careful interpretation of composite primary outcomes and the important role secondary outcomes can play in interpreting results.2 Because thoughtful assessment of secondary end points can reveal treatment implications that may otherwise be hidden within the primary (often composite) trial end points, the FIRE AND ICE Trial was predefined to evaluate secondary measures of clinical success. These secondary end points were designed to capture insight into the patient‐felt burden of AF after cryoballoon or RFC ablation and identified significant differences between treatment cohorts in measures of clinical success for both patients and healthcare systems.3, 4
The secondary outcomes advanced our understanding of the patient‐felt impact of AF recurrence after ablation, but important outstanding questions remain. To identify critical evidence gaps that need to be addressed, this review first comprehensively summarizes the published data from the FIRE AND ICE Trial. It then provides additional analyses to evaluate the influence of events that occurred during the blanking period on the overall patient burden of AF recurrence and the impact of newly introduced technology on the trial results. Finally, we discuss outstanding questions and anticipated upcoming trials aimed at closing knowledge gaps to ultimately guide and optimize treatment strategies across the AF disease continuum.
What We Know From the FIRE AND ICE Trial
Primary Efficacy and Safety
The FIRE AND ICE Trial tested noninferiority between cryoballoon (Arctic Front or Arctic Front Advance; Medtronic, Inc) and RFC (ThermoCool, ThermoCool SF, or ThermoCool SmartTouch; Biosense Webster, Inc) ablation for PVI in a large, randomized cohort of patients with drug‐refractory paroxysmal AF.1 The primary efficacy end point was assessed by way of a time‐to‐first‐event analysis, outside the landmark 90‐day blanking period (during which, recurrence[s] of atrial arrhythmias were not counted against the primary end point), of the following prespecified failure events: (1) documented recurrence of AF >30 seconds, atrial tachycardia, or atrial flutter; (2) prescription of antiarrhythmic drugs; and (3) repeat catheter ablation. The Kaplan‐Meier primary efficacy event‐rate estimates at 12 months after the index procedure were 34.6% in the cryoballoon cohort and 35.9% in the RFC cohort, confirming noninferiority between the ablation modalities (P<0.001).1
This trial also identified no difference in the primary safety end point between treatment cohorts (P=0.24).1 Although there was no statistical difference in the absolute number of patients who reached the primary safety end point, there were differences in the type of safety events that occurred in cryoballoon‐ versus RFC‐treated patients. Specifically, phrenic nerve injury at discharge was reported more often in the cryoballoon (2.7%) than in the RFC treatment cohort (0%; P=0.001), and there was a trend for more groin site complications in the RFC cohort than in the cryoballoon cohort (4.3% versus 1.9%; P=0.09).1 The occurrence of atrial flutter or atrial tachycardia after catheter ablation of AF is well documented, and although debated, it is thought to be created (in part) by incomplete lesions or gaps in AF ablation lines that develop into a new substrate for a reentry circuit.5 For this reason, occurrences of atrial flutter and atrial tachycardia were also included as a serious adverse event. It was reported that atrial flutter and atrial tachycardia tended to be more prevalent in the RFC cohort versus the cryoballoon cohort (10/376 [2.7%] versus 3/374 [0.8%]; P=0.09).1 This observation may suggest that newly arrhythmogenic tissue, potentially driven by incomplete PVI or tissue heterogeneity, tends to be created more frequently after an index RFC ablation than an index cryoablation.
Secondary Outcomes
Because the FIRE AND ICE Trial was a large trial and the primary end point hypothesis was met, it was both appropriate and important to evaluate this data set further with respect to secondary outcomes.2 Predefined secondary end points in the trial were designed to compare procedure and fluoroscopy times between the 2 cohorts as well as the patient‐felt disease burden of AF through quality of life, cardiovascular rehospitalizations, and repeat ablations. Mean total procedure and left atrial dwell durations were significantly shorter in the cryoballoon cohort, whereas mean fluoroscopy time was significantly shorter in the RFC cohort.1 As per the Short Form 12 and the EuroQol 5‐dimension form, the treatment groups experienced similar improvements in mental and physical measures of quality of life by 6 months, which were maintained in both groups throughout follow‐up.3 Secondary analyses also revealed that the cryoballoon cohort (versus RFC) had significantly fewer all‐cause rehospitalizations (32.6% versus 41.5%; P=0.01), cardiovascular rehospitalizations (23.8% versus 35.9%; P<0.01), repeat ablations (11.8% versus 17.6%; P=0.03), and direct current cardioversion after the index procedure (3.2% versus 6.4%; P=0.04).3
The reduced need for subsequent medical treatment after the index ablation procedure in the cryoballoon cohort was a clinical result illustrating the generalized impact that AF disease burden imposes on current healthcare systems. A trial‐period economic analysis revealed lower resource use in the cryoballoon treatment group (205 healthcare uses in 122 of 374 patients) compared with the RFC treatment group (268 healthcare uses in 154 of 376 patients).4 This reduction in resource use was modeled to translate into significantly reduced patient and overall healthcare expenditures across 3 distinct healthcare systems (ie, Germany, United Kingdom, and United States).4
For Which Patients Is Catheter Ablation Challenging?
Regardless of the catheter ablation modality, 37% (281/750) of patients treated in the trial reached the primary efficacy end point.1 The reasons for individual patient failure and the optimal strategy to treat patients for whom PVI is inadequate remain largely unknown. To inform treatment strategy, a multivariable regression analysis was performed to identify baseline characteristics that predict poor outcomes after catheter ablation of paroxysmal AF, regardless of the treatment modality. Of 22 baseline patient characteristics, female sex was a strong, independent predictor of the primary end point and of cardiovascular rehospitalization.6 Baseline characteristics that were indicative of a longer cardiac disease progress (previous direct current cardioversion, hypertension, and longer duration of AF) were also independently associated with poorer clinical outcomes,6 but it is unclear how these baseline characteristics impede clinical success. To better understand the impact of treatment paradigms on patient outcomes, we returned to the study data set to assess the influence of the 90‐day blanking period on patient burden of arrhythmia recurrence and to assess the effect of technological advancement on trial results.
What We Can Still Learn: Patient‐Felt Burden of AF Disease and the Blanking Period
Time‐to‐first event analyses can be critical to a robust clinical study because they are designed to restrict the influence of oversampling bias by a few highly symptomatic subjects in a larger patient cohort; however, the major limitation of this clinical study design in real‐world patient application is that it fails to reveal the sequalae a patient experiences after the clinical trial end point. The “patient burden” of AF recurrence after an index ablation is not limited to the first event, but rather is defined by the summation of the number, type, and severity of events that follow. As illustrated, a patient without any AF recurrence after an index procedure is deemed a clinical trial success (Figure 1) and likely lives a lifestyle reflective of that freedom from atrial arrhythmia. By comparison, both a patient with an isolated recurrence and a patient with frequent recurrences are equally deemed clinical trial failures by time‐to‐first event analysis (Figure 1). However, in the real world, the patient with frequent recurrences is burdened by more episodes of atrial arrhythmias than the patient with an isolated recurrence, and, therefore, the former patient is more likely to undergo a future cardioversion, rehospitalization, and/or repeat ablation. Arrhythmias that lead to emergency department triage and rehospitalization should not be ignored (or remain uncounted) because of a time‐to‐first‐event clinical study design.
Figure 1.
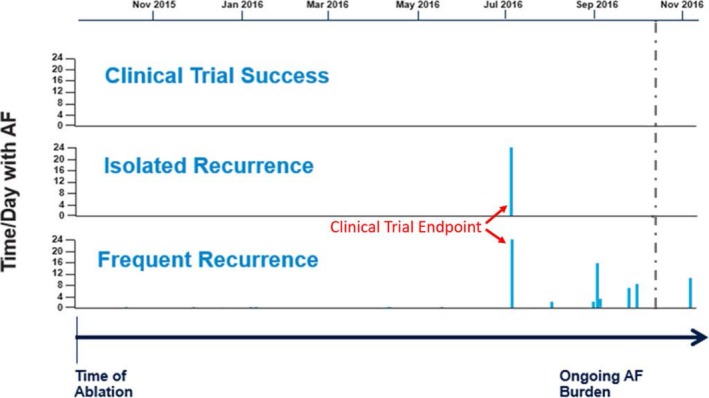
Clinical trial success and failure vs patient burden of atrial fibrillation (AF) recurrence. Clinical trial success is illustrated in the top panel by the absence of AF recurrence events from the time of the index ablation through the end of the study period indicated by the vertical, dashed line. The middle panel demonstrates a patient who reached the clinical trial end point via an isolated AF recurrence, indicated by the single, vertical solid line within the study period. The bottom panel represents a patient who reached the clinical trial end point and subsequently experienced multiple AF recurrence events after the initial recurrence, which is demonstrated by the collection of vertical, solid lines over time. Critically, both patients in the middle and bottom panel initially recurred at the same time point during the clinical trial; therefore, these 2 patients are counted equally in the primary efficacy end point, although the burden of AF that followed the initial recurrence drastically differs between the 2 patients.
The distinction between “clinically free of AF” and “meaningfully free of AF” is increasingly recognized,7, 8 and future studies will define patient disease burden and healthcare burden while still collecting traditional clinical study measurements of AF burden. In the FIRE AND ICE Trial, predefined secondary outcome measurements were rigorously collected and analyzed with the intention to explore patient and healthcare arrhythmia burden. Although the trial was not designed to assess AF burden (ie, the percentage of time a patient was in AF), the predefined secondary outcomes elucidated the impact of 2 different catheter ablation treatments on patient‐felt AF disease burden via measures of quality of life, repeat ablations, and cardiovascular rehospitalizations, which one may infer are the outcome measures of AF disease burden.
The secondary analysis was predefined to include events that occurred during the blanking period to capture the total patient and healthcare burden of AF recurrence between the 2 cohorts. However, for this current review of study data, cardiovascular rehospitalization and repeat ablations that occurred during the 90‐day blanking period were excluded to reveal the impact of these early events on the overall conclusions (Figure 2). This additional analysis demonstrated that there were significantly fewer cardiovascular rehospitalizations in the cryoballoon cohort whether or not events in the 90‐day blanking period were included in the analysis (P<0.01 and P=0.02, respectively; Figure 2A). RFC catheter ablation was associated with a significantly higher risk of cardiovascular rehospitalization regardless of the events that occurred within the first 90 days after the index procedure. The number of total hospitalizations and the number of subjects hospitalized in each cohort are detailed in Table 1. By contrast, freedom from repeat ablation was no longer statistically significant when repeat ablations that occurred within the 90‐day blanking period were removed from the analysis (P=0.03 versus P=0.10; Figure 2B). The numbers of repeat ablations in the cryoballoon and RFC cohorts are presented in Table 2.
Figure 2.
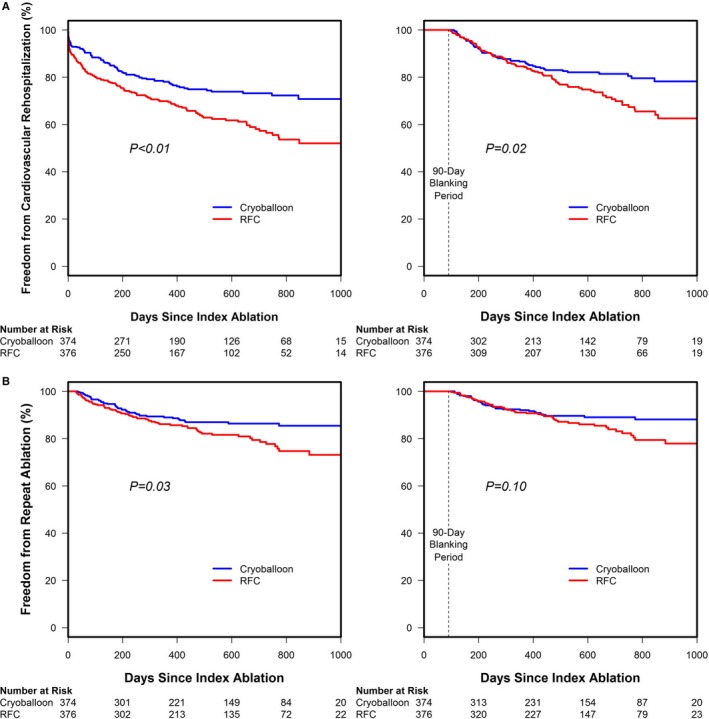
Survival free from predefined secondary end points, including and excluding events that occurred during the 90‐day blanking period (modified intention‐to‐treat cohort). A, Freedom from cardiovascular rehospitalization with and without a 90‐day blanking period in cryoballoon‐ vs radiofrequency current (RFC)–treated cohorts is presented. Freedom from cardiovascular rehospitalization was significantly higher in the cryoballoon cohort both when events in the blanking period were included in the analysis (log‐rank test, P<0.01) and when they were not used (log‐rank test, P=0.02). B, Freedom from repeat ablation in the cryoballoon vs RFC cohort with and without the blanking period is compared. Freedom from repeat ablation was significantly higher in the cryoballoon cohort when events in the blanking period were included in the analysis (log‐rank test, P=0.03), but the cohorts were no longer statistically different when events in the blanking period were excluded (log‐rank test, P=0.10).
Table 1.
Cardiovascular Rehospitalizations Within and Beyond the Blanking Period by Cohort
| Randomization Arm | Time of Cardiovascular Rehospitalization | No. of Rehospitalizations (No. of Subjects; % of Subjects) |
|---|---|---|
| Cryoballoon | Total | 139 (89; 23.8) |
| Within blanking period | 48 (42; 11.2) | |
| Beyond blanking period | 91 (59; 15.8) | |
| Radiofrequency current | Total | 203 (135; 35.9) |
| Within blanking period | 83 (71; 18.9) | |
| Beyond blanking period | 120 (86; 22.9) |
Table 2.
Repeat Ablations Within and Beyond the Blanking Period by Cohort
| Randomization Arm | Time of the Repeat Ablation | No. of Repeat Ablations (No. of Subjects; % of Subjects) |
|---|---|---|
| Cryoballoon | Total | 49 (44; 11.8) |
| Within blanking period | 12 (12; 3.2) | |
| Beyond blanking period | 37 (34; 9.1) | |
| Radiofrequency current | Total | 70 (66; 17.6) |
| Within blanking period | 19 (19; 5.1) | |
| Beyond blanking period | 51 (49; 13.0) |
With or without the blanking period, the observed difference in freedom from cardiovascular rehospitalization and repeat ablation between cohorts continues to diverge beyond the first year of follow‐up (Figure 2). These data may suggest that, although the timing of initial recurrence was equivalent between cohorts, the symptoms and severity of recurrence felt by patients treated with RFC necessitated increased intervention over time.
What We Can Still Learn: Impact of New Technology
Over the course of the trial, technological advancements in both cryoballoon and RFC ablation catheters were introduced into the commercial market and incorporated into the study at the discretion of the investigators. In the cryoballoon arm, Arctic Front ablation catheters were denoted as the first‐generation cryoballoon, and Arctic Front Advance catheters were labelled as the second‐generation cryoballoon. Similarly, in the RFC arm, ThermoCool and ThermoCool SF were categorized as the first‐generation RFC catheters, whereas the contact‐force sensing ThermoCool SmartTouch catheters were denoted as the advanced‐generation RFC ablation catheters. New catheter technology was not equally adopted into the trial; the second‐generation cryoballoon was used in 75.6% of patients in the cryoballoon arm, whereas advanced‐generation RFC catheters were used in only 24.7% of patients in the RFC group.1 The disproportionate use of second‐generation cryoballoon versus advanced‐generation RFC catheters may have obscured the efficacy of the advanced‐generation RFC contact‐force sensing technology. Although there was no difference in the primary efficacy outcome between catheter generations,1 it is imperative to understand the influence of newly introduced technology on the comprehensive clinical outcomes as new technologies inundate the field of AF ablation.
Safety and Procedural Outcomes by Catheter Generation
Additional analyses according to catheter subtype using an as‐treated cohort consistent with prior publications were performed (Figure 3). Importantly, this revealed no difference in the primary safety end point between catheter subtypes (P=0.44; Figure 4). Analyses of procedural data indicated that total procedure and left atrial dwell times were significantly shorter for second‐generation cryoballoon‐treated patients compared with the first‐generation cryoballoon‐ or any‐generation RFC ablation catheter‐treated cohort (P<0.01), whereas the mean procedure and left atrial dwell times were unchanged between first‐ and advanced‐generation RFC procedures (Table 3). Use of the second‐generation cryoballoon reduced the fluoroscopy exposure time compared with patients treated with the first‐generation cryoballoon (20±13 versus 27±15 minutes). However, the fluoroscopy time for the second‐generation cryoballoon remained higher than for the first‐generation RFC (17±19 minutes) or the advanced‐generation RFC (17±12 minutes) treated cohorts (Table 3).
Figure 3.
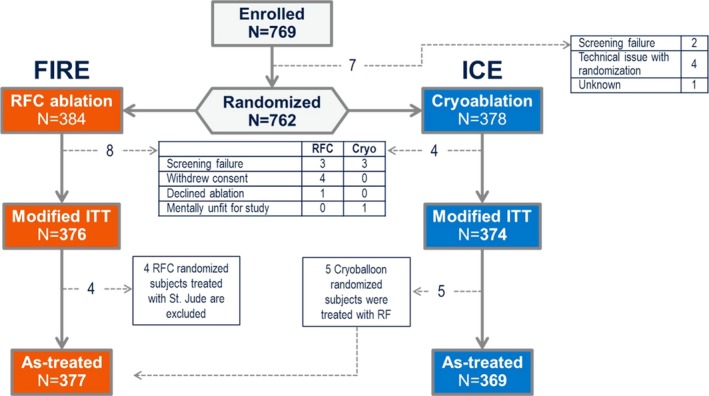
Flowchart of patient cohort assignment in the FIRE AND ICE Trial. This figure depicts the total number of patients randomized, the total number of patients in the modified intention‐to‐treat (ITT) cohort, and the patients who composed the as‐treated cohorts used for analyses of subcatheter differences. Cryo indicates cryoballoon; RFC, radiofrequency current.
Figure 4.
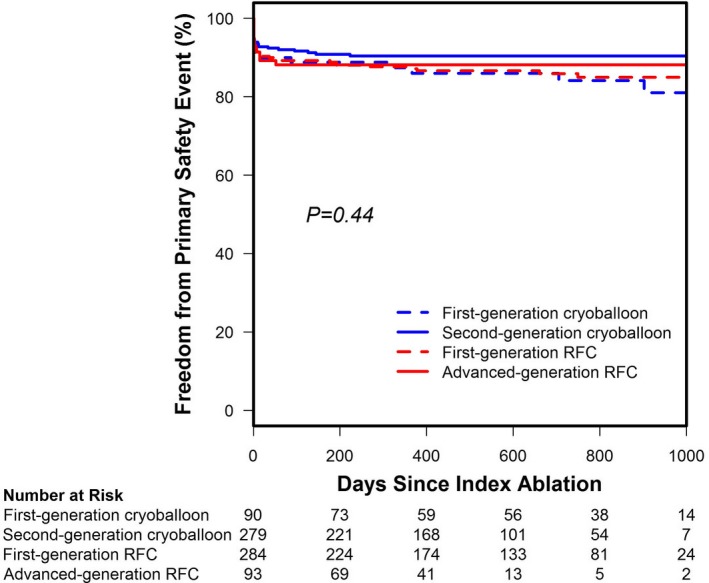
Survival free from a primary safety event by catheter subtype. An across‐group analysis of the as‐treated cohort revealed there was no statistical difference in the risk of a primary safety event across catheter subtypes (log‐rank test, P=0.44). RFC indicates radiofrequency current.
Table 3.
Procedural Data by Catheter Type
| Parameter | First‐Generation Cryoballoon (N=90) | Second‐Generation Cryoballoon (N=279) | First‐Generation RFC (N=284) | Advanced‐Generation RFC (N=93) | P Value |
|---|---|---|---|---|---|
| Acute PVIa | 99.2 (351/354) | 98.7 (1107/1122) | 98.2 (1134/1155) | 97.1 (362/373) | 0.13b |
| Acute left common PVIa | 100 (10/10) | 100 (16/16) | 74.2 (23/31)c | 90.0 (9/10) | 0.04b |
| Procedure time, min | 140±32 | 118±38 | 141±57 | 143±50 | <0.01d |
| Left atrial dwell time, min | 101±32 | 89±29 | 109±47 | 109±41 | <0.01d |
| Fluoroscopy time, min | 27±15 | 20±13 | 17±19 | 17±12 | <0.01d |
Data are given as mean±SD unless otherwise indicated. PVI indicates pulmonary vein isolation; RFC, radiofrequency current.
Data are given as percentage (number/total). These were treated/targeted pulmonary veins.
P value from exact test.
RF randomization arm treated with ThermoCool: 21/29; cryoballoon randomization arm treated with ThermoCool: 2/2.
P value from ANOVA.
The overall rate of acute PVI approached 100% regardless of the ablation modality used (P=0.13; Table 3). However, there were catheter‐dependent differences in the rate of acute PVI during treatment of left common pulmonary veins (LCPVs; P=0.04; Table 3). Specifically, 100% of LCPVs were isolated with the first‐generation (10/10) and second‐generation (16/16) cryoballoon. By contrast, 74.2% (23/31) of LCPVs were isolated with the first‐generation RFC ablation catheter, which improved to an acute isolation rate of 90.0% (9/10) with use of the advanced‐generation RFC ablation catheter. Of the 31 LCPVs ultimately treated with RFC, 2 were from patients initially randomized to cryoballoon ablation (both of whom experienced successful acute isolation with the first‐generation RFC ablation catheter). Together, these data suggest that LCPVs are frequently isolated regardless of the catheter ablation technology. While RFC ablation catheters are inherently flexible during lesion application in uncommon anatomical features, high rates of LCPV isolation via the cryoballoon are likely enabled by a segmental approach to circumferential isolation of the LCPV ostium.9
Predefined Secondary Outcomes by Catheter Subtype
To better understand the influence of the ablation catheter generation on predefined secondary outcomes, freedom from cardiovascular rehospitalization and repeat ablation was analyzed by ablation catheter subtype (Figure 5). This analysis revealed a significant difference in freedom from cardiovascular rehospitalization between catheter types (P<0.01). Both first‐ and second‐generation cryoballoon cohorts were associated with higher rates of freedom from cardiovascular rehospitalization compared with either the first‐ or advanced‐generation RFC cohorts (Figure 5A). Notably, patients treated with the second‐generation cryoballoon experienced a higher rate of freedom from cardiovascular rehospitalization than those treated with the first‐generation cryoballoon. By contrast, freedom from cardiovascular rehospitalization was similar for the first‐ and advanced‐generation RFC catheters (Figure 5A). Although there was a difference in freedom from cardiovascular rehospitalization between catheter subtypes, there was no significant difference in the rates of repeat ablation between the 4 catheters (Figure 5B).
Figure 5.
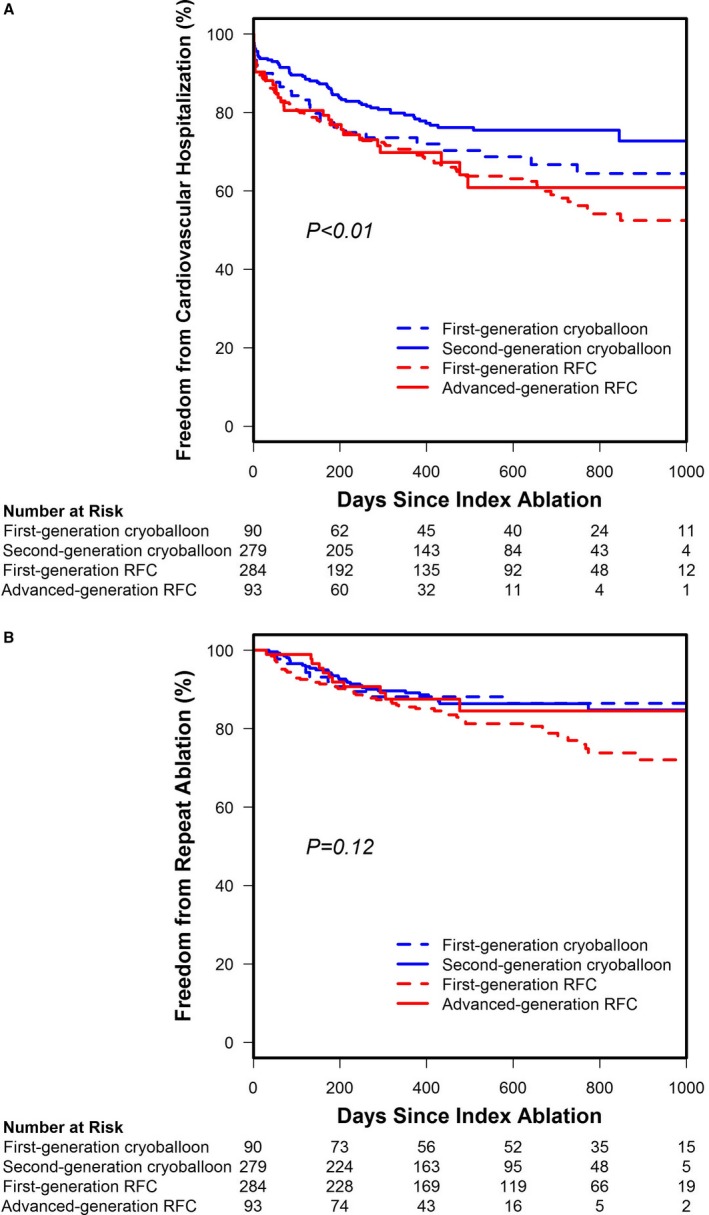
Freedom from predefined secondary end points by catheter subtype in the as‐treated cohort. A, Freedom from cardiovascular rehospitalization by catheter subtype is displayed; there was a significant difference between catheter types (log‐rank test, P<0.01). B, The freedom from repeat ablation across catheter subtypes in the as‐treated cohort. There was no difference in the rate of repeat ablation when examined by catheter type (log‐rank test, P=0.12). RFC indicates radiofrequency current.
What We Need to Address in the Future
As with all successful trials, the FIRE AND ICE Trial generated many questions and future directions for potential clinical research. Specifically, pressing questions persist in the categories of repeat ablations, persistent AF, and newer ablation catheter technologies.
The FIRE AND ICE Redo Study
A substantial proportion of patients experienced recurrence of AF (37% primary efficacy failure in the FIRE AND ICE Trial) after an initially successful PVI. In these patients, re‐isolation of the PVs may be warranted. Little is known about redo ablation after PVI, particularly in regard to electrophysiological differences after an initial cryoballoon or RFC procedure. During a follow‐up of maximally 33 months, repeat ablations were performed in 12% (n=44) of patients who had undergone an index PVI with the cryoballoon and 18% (n=66) of patients after an index PVI using RFC.
Recently, 89 of the 110 FIRE AND ICE Trial patients with repeat ablations were retrospectively consented and enrolled in a redo study (ClinicalTrials.gov, identifier NCT03314753) with the intention to evaluate lesion durability, repeat ablation strategy, and procedural characteristics in those trial patients. There were 36 patients originally randomized to cryoballoon ablation and 53 patients randomized to RFC ablation. These repeat ablation data are currently being analyzed and will be published shortly.
The FIRE AND ICE II Randomized Trial
AF is progressive in nature, and paroxysmal AF evolves into persistent AF with an overall rate of 5.5% per year.10 AF can initiate irreversible fibrosis at many cardiac sites.11 With more atrial fibrosis, subjects with AF are increasingly likely to experience less favorable outcomes.12 The clinical implications of this association warrant further investigation.
There is a need for adequately powered randomized trials evaluating catheter ablation in subjects with persistent, as opposed to paroxysmal, AF. The FIRE AND ICE II randomized outcome trial has been designed to compare the efficacy and safety of PVI using cryoballoon versus RFC ablation with a contact‐force sensing catheter in subjects with persistent AF. The primary objective of the trial is to demonstrate that cryoballoon ablation is noninferior to RFC ablation with respect to the time‐to‐first clinical failure, defined as recurrence of atrial arrhythmias or intervention for AF (a blanking period of 90 days will be maintained after the index procedure), which maintains study design congruence with historical trials so that the data can be compared. Intervention for AF will include hospitalizations, cardioversions, repeat ablations, and new antiarrhythmic subscriptions given for the treatment of AF. Furthermore, on the basis of magnetic resonance imaging, the trial will investigate the impact of left atrial volume and left atrial fibrosis on clinical outcome and assess lesion formation 3 months after ablation. In addition, the impact of PVI on electrical sources identified by body surface mapping will be investigated.
The Potentially Revolutionary Ablation Modality: Pulsed Electrical Field Ablation
In the next decade, this cryoballoon versus RFC series of trials may only serve to be a historical reference point as newer or newly redesigned ablation energies and catheters enter the commercial market space. The “novel” pulsed electrical field (PEF) ablation technology13 is (in fact) a revival of an old technology (namely, electroporation) that was used in the early days of catheter ablation at different energy settings, largely with high voltages delivered in a single pulse. PEF ablation uses similarly high voltages (ie, 900–2500 V), but they are delivered in multiple ultrarapid millisecond pulses. The mode of action is unique in that this energy ablates nonthermally by creating nanoscale pores in cell membranes. PEF energy is characterized by tissue‐specific thresholds, thereby preferring ablation of myocardial tissue, which has the lowest threshold for PEF ablation. Therefore, in contrast to any other energy source, this energy spares collateral structures, such as the esophagus, arteries, and nerves.
In a small series of 22 patients with AF,13 PEF ablation was successfully applied to isolate pulmonary veins (100%) endocardially with a catheter‐based approach and to create a box lesion epicardially during cardiac surgery (86%) with a rather low number of energy applications (3.3±0.5 lesions/PV and 2 lesions/patient). No complications were noted. If these initial results can be verified and reproduced by other independent investigators, including proof of no or “little” PV reconnection over time, other available ablation technologies may no longer need to be refined to improve the results in ablation of AF.
Summary
In 2012, the year the FIRE AND ICE Trial was initiated, the European Society of Cardiology and the Heart Rhythm Society consensus statements recognized RFC as the dominant energy source for AF catheter ablation and discussed PVI as a promising treatment approach.14, 15 In response to the FIRE AND ICE Trial and many other trials, the consensus documents were updated to recognize PVI as a safe and effective strategy for the treatment of AF with either of the 2 leading catheter ablation modalities, cryoballoon or RFC ablation.16, 17 Evaluation of the comprehensive end points, in addition to the primary end point that demonstrated noninferiority between the treatment modalities, may have contributed to the acceptance and adoption of cryoballoon ablation. Indeed, secondary end points of the trial were critical in identifying clinically important differences between the 2 treatment cohorts. Thoughtful design and rigorous assessment of secondary outcomes of future clinical trials may be essential to defining the patient‐specific impact of new catheter ablation technologies for the treatment of AF.
Sources of Funding
The FIRE AND ICE Trial was funded by Medtronic, Inc.
Disclosures
Kuck reports personal fees from Medtronic and Biosense Webster during the conduct of the study and personal fees from St Jude Medical outside the submitted work. Braegelmann and Kueffer are employees of Medtronic. Chun reports grant support and personal fees from Medtronic during the conduct of the study and personal fees from Biosense Webster outside the submitted work. Albenque reports personal fees from St Jude Medical and Biosense Webster outside the submitted work. Calkins reports personal fees from Medtronic, Biosense Webster, and Abbott Medical outside the submitted work. The remaining authors have no disclosures to report.
The FIRE AND ICE Trial Investigators are as follows: Karl‐Heinz Kuck, Andreas Metzner, Feifan Ouyang (Asklepios Klinik St Georg, Hamburg, Germany); Julian Chun, Alexander Fürnkranz (Cardioangiologisches Centrum Bethanien, Frankfurt, Germany); Arif Elvan (Isala Klinieken Zwolle, the Netherlands); Thomas Arentz (Herz‐Zentrum Bad Krozingen, Germany); Michael Kühne, Christian Sticherling (Universitätsspital Basel, Switzerland); Laszlo Gellér (Semmelweis Egyetem, Budapest, Hungary); Matthias Busch (Uniklinik Greifswald, Germany); Josep Brugada, Lluis Mont (Hospital Clinic de Barcelona, Spain); Alberto Barrera (Hospital Clínico Universitario “Virgen de la Victoria” Malaga, Spain); Thomas Deneke (Klinikum Bad Neustadt, Germany); Jean‐Paul Albenque (Clinique Pasteur Toulouse, France); Volker Kühlkamp (Herz‐Zentrum Bodensee, Germany); Claudio Tondo (Centro Cardiologico Monzino, University of Milan, Italy); Ricardo Ruiz‐Granell (Hospital Clinico Universitario Valencia, Spain); Peter Neuzil (NA Homolce Hospital Prague, Czech Republic); Nicasio Pérez‐Castellano (Hospital Clinico San Carlos, Madrid, Spain).
(J Am Heart Assoc. 2018;7:e010777 DOI: 10.1161/JAHA.118.010777.)
Contributor Information
Karl‐Heinz Kuck, Email: kuckkh@aol.com.
on behalf of the FIRE AND ICE Trial Investigators:
Andreas Metzner, Feifan Ouyang, Julian Chun, Alexander Fürnkranz, Arif Elvan, Thomas Arentz, Michael Kühne, Christian Sticherling, Laszlo Gellér, Matthias Busch, Lluis Mont, Alberto Barrera, Thomas Deneke, Volker Kühlkamp, Ricardo Ruiz‐Granell, Peter Neuzil, and Nicasio Pérez‐Castellano
References
- 1. Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KR, Elvan A, Arentz T, Bestehorn K, Pocock SJ, Albenque JP, Tondo C; FIRE AND ICE Investigators . Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–2245. [DOI] [PubMed] [Google Scholar]
- 2. Pocock SJ, Stone GW. The primary outcome is positive: is that good enough? N Engl J Med. 2016;375:971–979. [DOI] [PubMed] [Google Scholar]
- 3. Kuck KH, Fürnkranz A, Chun KR, Metzner A, Ouyang F, Schlüter M, Elvan A, Lim HW, Kueffer FJ, Arentz T, Albenque JP, Tondo C, Kühne M, Sticherling C, Brugada J; FIRE AND ICE Investigators . Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, hospitalization, and quality‐of‐life outcomes in the FIRE AND ICE trial. Eur Heart J. 2016;37:2858–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chun KRJ, Brugada J, Elvan A, Gellér L, Busch M, Barrera A, Schilling RJ, Reynolds MR, Hokanson RB, Holbrook R, Brown B, Schlüter M, Kuck KH; FIRE AND ICE Investigators . The impact of cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation on healthcare utilization and costs: an economic analysis from the FIRE AND ICE trial. J Am Heart Assoc. 2017;6:e006043 DOI: 10.1161/JAHA.117.006043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sághy L, Tutuianu C, Szilágyi J. Atrial tachycardias following atrial fibrillation ablation. Curr Cardiol Rev. 2015;11:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuck KH, Brugada J, Fürnkranz A, Chun KRJ, Metzner A, Ouyang F, Schlüter M, Elvan A, Braegelmann KM, Kueffer FJ, Arentz T, Albenque JP, Kühne M, Sticherling C, Tondo C; FIRE AND ICE Investigators . Impact of female sex on clinical outcomes in the FIRE AND ICE Trial of catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2018;11:e006204. [DOI] [PubMed] [Google Scholar]
- 7. Steinberg JS, O'Connell H, Li S, Ziegler PD. Thirty‐second gold standard definition of atrial fibrillation and its relationship with subsequent arrhythmia patterns: analysis of a large prospective device database. Circ Arrhythm Electrophysiol. 2018;11:e006274. [DOI] [PubMed] [Google Scholar]
- 8. Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, Noseworthy PA, Perez MV, Turakhia MP; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council . Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation. 2018;137:e623–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Su W, Aryana A, Passman R, Singh G, Hokanson R, Kowalski M, Andrade J, Wang P. Cryoballoon best practices II: practical guide to procedural monitoring and dosing during atrial fibrillation ablation from the perspective of experienced users. Heart Rhythm. 2018;15:1348–1355. [DOI] [PubMed] [Google Scholar]
- 10. Kato T, Yamashita T, Sagara K, Iinuma H, Fu LT. Progressive nature of paroxysmal atrial fibrillation: observations from a 14‐year follow‐up study. Circ J. 2004;68:568–572. [DOI] [PubMed] [Google Scholar]
- 11. Shinagawa K, Shi YF, Tardif JC, Leung TK, Nattel S. Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation. 2002;105:2672–2678. [DOI] [PubMed] [Google Scholar]
- 12. Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 13. Reddy VY, Koruth J, Jais P, Petru J, Timko F, Skalsky I, Hebeler R, Labrousse L, Barandon L, Kralovec S, Funosako M, Mannuva BB, Sediva L, Neuzil P. Ablation of atrial fibrillation with pulsed electric fields: an ultra‐rapid, tissue‐selective modality for cardiac ablation. JACC Clin Electrophysiol. 2018;4:987–995. [DOI] [PubMed] [Google Scholar]
- 14. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines (CPG) . 2012 Focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation: developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 15. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171–257. [DOI] [PubMed] [Google Scholar]
- 16. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: the Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC endorsed by the European Stroke Organisation (ESO). Europace. 2016;37:2893–2962. [Google Scholar]
- 17. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d'Avila A, de Groot NMSN, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ, Yamane T. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]


