Figure 4.
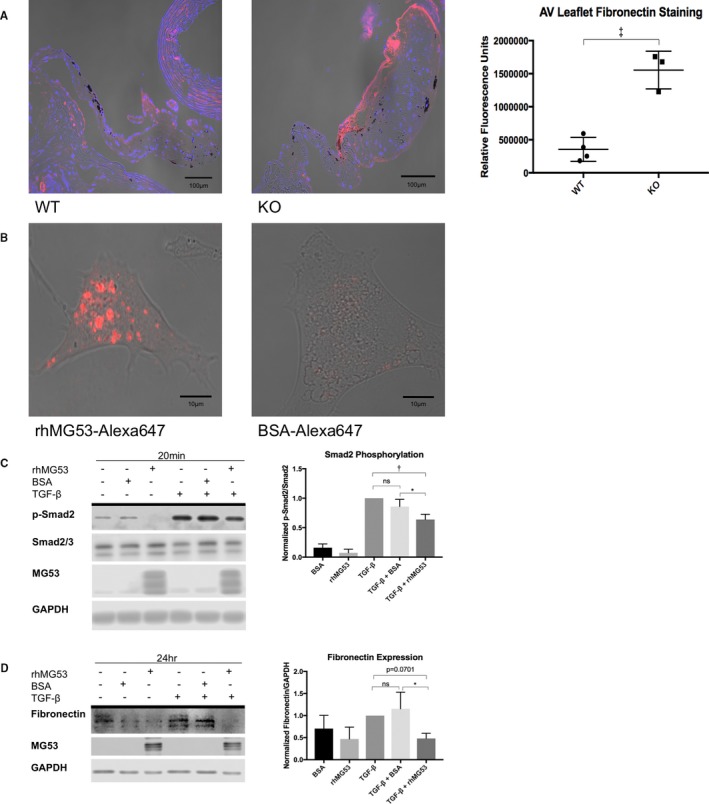
rhMG53 suppresses TGF‐β‐induced fibrocalcific signaling in valve interstitial cells. A, Immunohistochemistry of aged wild‐type (left) and Mg53−/− (right) mouse hearts show increased fibronectin staining in Mg53−/− aortic valve leaflets (n=3) compared with those from wild‐type control mice (n=4). A 2‐tailed Student t test was used to obtain the P value. In different tissues, fibronectin mediates both fibrosis and calcification such as that observed in histologic analysis of 24‐month‐old Mg53−/− mouse valves (Figure 2).41, 42, 43, 44 Fibronectin is a downstream product of the TGF‐ß pathway and has been associated with VIC injury, facilitating continued signaling events and VIC activation.7, 8, 45, 48, 49, 50, 57 Given the relationship between TGF‐ß and fibronectin and with increased fibronectin staining in Mg53−/− aortic valves, we aimed to target our in vitro studies through our histopathology findings. Specifically, we tested if rhMG53 treatment could modulate VIC TGF‐ß signaling and downstream fibronectin expression. B, To guide our mechanistic insights, we first wanted to determine if rhMG53 could enter pig aortic VICs. Here, we conjugated rhMG53 and BSA as a control to the Alexa‐647 fluorophore to allow visualization of rhMG53‐specific cell entry via live cell imaging (n=10 cells). Importantly, rhMG53 VIC entry was observed after treating cells for 30 minutes, thus our use of this pretreatment time in our in vitro signaling studies. C, Short time points to study cell signaling events in pig VICs showed that rhMG53 reduces TGF‐β‐induced Smad2 phosphorylation (n=3). D, Longer time points to study differences in protein expression in pig VICs demonstrated that rhMG53 reduces TGF‐β‐induced fibronectin expression (n=4). Ordinary, 1‐way ANOVA tests with Tukey multiple comparison testing (α=0.05) were used to obtain P‐values. AV indicates aortic valve; BSA, bovine serum albumin; KO, knockout (Mg53−/−); rh, recombinant human; TGF, transforming growth factor; WT, wild type. *P<0.05, **P<0.01, ***P=0.001.
