Figure 8.
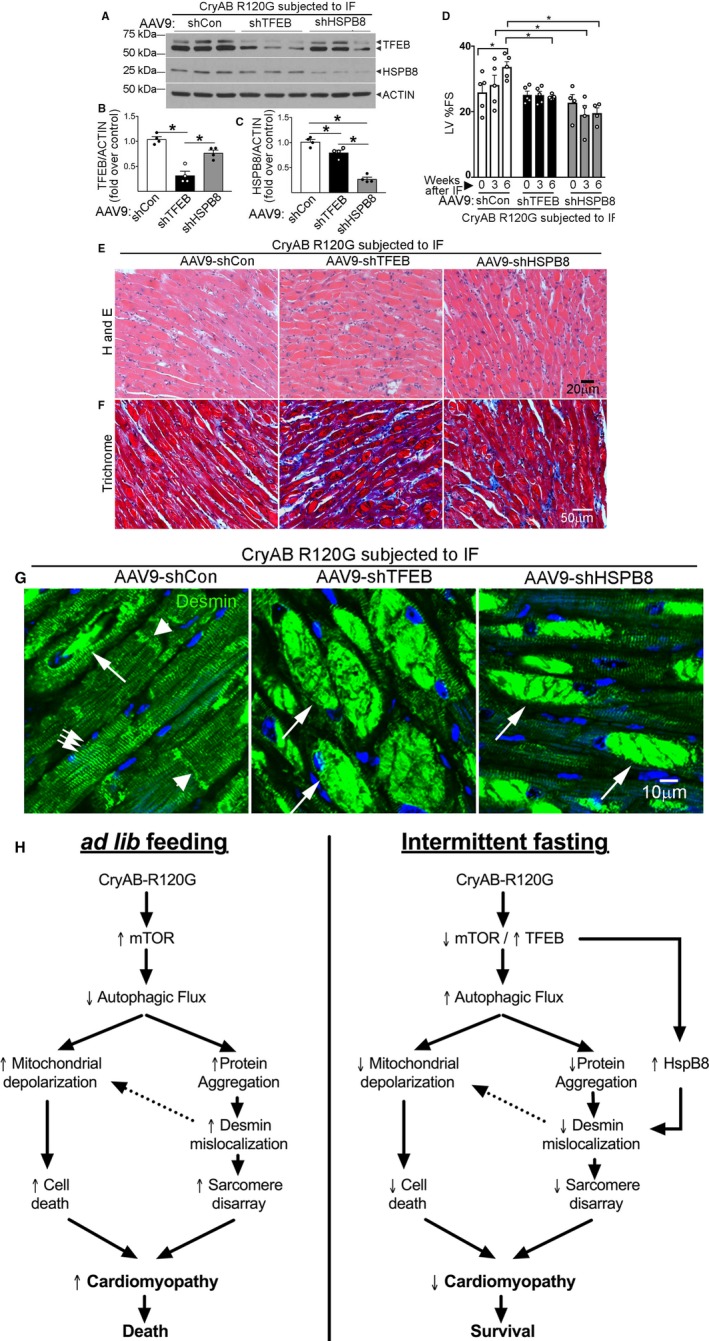
Knockdown of transcription factor EB (TFEB) and HSPB8 prevents intermittent fasting (IF)–mediated attenuation of proteotoxic cardiomyopathy with persistent desmin localization to aggregates. A through C, Representative immunoblot (A) with quantitation of TFEB expression (B) and HSPB8 abundance (C) in total cardiac protein extracts from Myh6‐CryABR120G transgenic mice transduced with adeno‐associated virus (AAV9)–short hairpin control (shCon), AAV9–short hairpin TFEB (shTFEB), or AAV9–short hairpin HSPB8 (shHSPB8) and subjected to IF, assessed at 46 weeks of age. N=4/group. *P<0.05 by post hoc test after 1‐way ANOVA. Please see schematic in Figure S12A for experimental design. D, Left ventricular percentage endocardial fractional shortening (LV %FS) in mice treated as in A and evaluated by echocardiography at baseline (4 weeks after injection of AAV9 particles and before IF, time=0 weeks) and 3 weeks and 6 weeks after initiating IF. N=4 to 5/group. *P<0.05 by post hoc test after 2‐way ANOVA. We did not observe mortality in these treatment groups. See Table S4 for additional echocardiographic data on these mice. E through G, Representative images demonstrating myocardial sections stained with hematoxylin‐eosin (H and E; E) and Masson's trichrome (F) and immunostained for desmin expression (G) in mice treated as in A. In G, white arrows in groups point to Z‐discs and arrowheads indicate intercalated discs to demonstrate desmin localization. Single long white arrows point to desmin localized in aggregates. H, Schematic depicting the mechanisms by which IF benefits advanced cardiomyopathy triggered by αB‐crystallin R120G mutation. Mutant R120G crystallin expression drives mammalian target of rapamycin (mTOR) activation with suppressed autophagic flux and aggregates sequestering desmin within the aggregates, thereby provoking mitochondrial abnormalities and cell death. IF suppresses mTOR activation, which activates TFEB to stimulate lysosome biogenesis and restores autophagic flux to remove mutant crystallin aggregates and relieve desmin sequestration. TFEB activation also stimulates HSPB8 expression, which chaperones desmin to its normal localization and restores mitochondrial quality to rescue cell death.
