Figure 2.
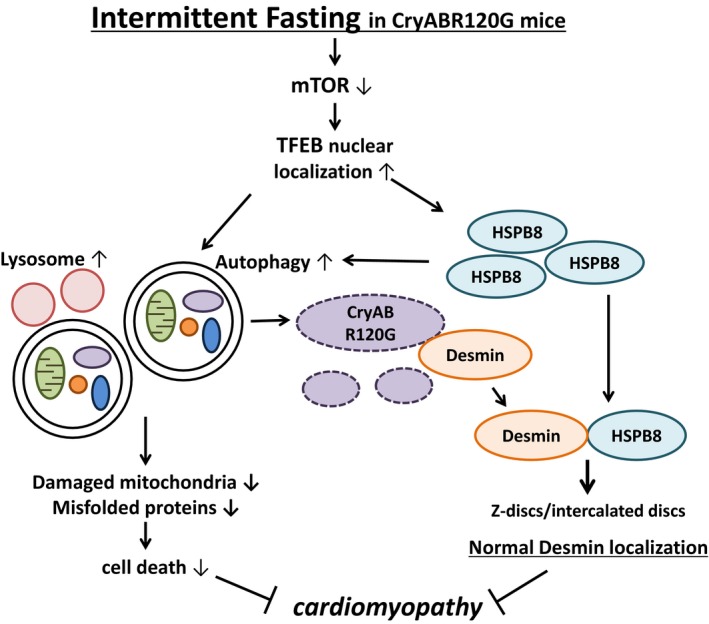
Molecular mechanisms through which intermittent fasting (IF) improves the cardiac phenotype in R120G αB‐crystallin (CryABR120G) mice. IF inactivates mammalian target of rapamycin (mTOR) and induces nuclear translocation of transcription factor EB (TFEB), a master regulator of lysosome biogenesis and autophagy. The salutary effect of IF is critically mediated by TFEB. TFEB not only activates autophagy, thereby eliminating protein aggregates and damaged mitochondria, but also transcriptionally upregulates heat shock protein (HSP) B8, which, in turn, normalizes localization of desmin. HSPB8 also stimulates autophagy through chaperone‐assisted selective autophagy and other unknown mechanisms. Autophagic degradation of protein aggregates frees desmin trapped inside, which, in turn, goes back to Z‐lines and intercalated discs with assistance of HSPB8 and allows restoration of the normal sarcomere structure. Although the study by Ma et al7 clearly showed that the salutary effects of TFEB in CryABR120G mice are critically mediated through HSPB8, whether stimulation of autophagy is indispensable remains to be clarified.
