Short abstract
Adipose and immune functions display sex differences and are influenced by sex steroid hormones in health and disease. However, effector molecules that mediate the effects of sex steroids and determine sex differences in adipocytes and immune cells are largely unknown. Sex differences are known to exist in mitochondrial biology, and mitochondria play a crucial role in adipocyte and immune cell functions. In fact, mitochondrial dysregulation is a common finding in a number of diseases that exhibit sex differences. It is, therefore, possible that mitochondria carry out sex-dimorphic functions. Prohibitin, an evolutionarily conserved pleiotropic protein, known to function as a mitochondrial chaperone, has multifaceted relationship with sex steroids and their receptors. New evidence indicates that prohibitin has roles in sex differences in multiple cell and tissue types, including adipocytes, macrophages, and dendritic cells. Transgenic mice overexpressing prohibitin in adipocytes, macrophages, and dendritic cells exhibit sex differences in metabolic and immune phenotypes, mediated through mitochondrial and plasma membrane signaling functions of prohibitin. Thus, the discovery of prohibitin as mediating the effects of sex steroids in multiple cell types has opened a new research direction to study the relationship between sex steroids and mitochondrial proteins and their impact on sex differences in health and disease. In this opinion article, we will provide a personal perspective of the role of prohibitin with cellular compartment- and tissue-specific functions in mediating sex-dimorphic adipose and immune functions. We believe that prohibitin is a potential target for sex-based new therapeutics for metabolic and immune diseases.
Impact statement
Traditional sex-related biases in research are now obsolete, and it is important to identify the sex of humans, animals, and even cells in research protocols, due to the role of sex as a fundamental facet of biology, predisposition to disease, and response to therapy. Genetic sex, epigenetics and hormonal regulations, generate sex-dimorphisms. Recent investigations acknowledge sex differences in metabolic and immune health as well as chronic diseases. Prohibitin, an evolutionarily conserved molecule, has pleotropic functions in mitochondrial housekeeping, plasma membrane signaling, and nuclear genetic transcription. Studies in adipocytes, macrophages, and transgenic mice indicate that prohibitin interacts with sex steroids and plays a role in mediating sex differences in adipose tissues and immune cell types. Prohibitin may, depending on context, modulate predisposition to chronic metabolic diseases and malignancy and, because of these attributes, could be a target for sex-based therapies of metabolic and immune-related diseases as well as cancer.
Keywords: Sex differences, epigenetics, mitochondria, sex steroids, X chromosome inactivation
Introduction
Susceptibility to disease is under the influence of numerous genetic, epigenetic, and hormonal factors, many of which may be inherent or specific to the sex of the individual. In humans and other mammals, the definition of sex has traditionally been based on external genitalia. This is supported by the presence of chromosome Y for males and its absence for females, and hormonally manifested by the predominance of testosterone and estrogens, respectively.1 Besides the organismal sex, it is more and more accepted that sex differences exist even at the cellular level, and mitochondrial factors have recently begun to be considered as contributing to sex differences.2,3 In clinical practice, sex differences in manifestations of diseases and their response to treatment have also been observed.4–7 Because of these biological and clinical differences between males and females, learned societies nowadays require that sex considerations be integrated in biomedical research, epidemiological data collections, and clinical trials.8,9 This would lead to better insights into mechanisms and clinical manifestations of diseases, with sex as an important variable.
In this perspective article, we have chosen adipose and immune functions as examples to discuss this viewpoint, because of their multifaceted role in health and disease as well as their intimate relationship with each other, which may have reciprocal influences on sex differences. Notably, an important source of metabolic and immunological variations is the sex of the individual. In general, females possess higher percentage of body fat, but display resistance to obesity-related metabolic dysregulation compared to males.10 This difference in metabolic function between females and males is attributed to sex differences in adipose tissue distribution in different fat depots and their functions.11 A parallel sex difference also exists in immune responses. For example, males experience a greater severity of various infections than females, whereas females exhibit a greater response to antigenic challenges such as infection and vaccination12,13 and are more prone to developing autoimmune diseases.14 Thus, there are fundamental aspects of metabolic homeostasis and immune functions that are regulated differently in males and females and likely influence both the development of metabolic and immune diseases and the response to pharmacological intervention.
As therapies targeting immune functions are developed to improve clinical outcomes in cancer, viral and bacterial infections, autoimmune diseases and transplantation, it is crucial for their success to identify the source of immunological variations and to find biomarkers for immune health and dysfunction.15 Some of the sex-specific variations in adipose tissue functions and immune responses may be directly attributed to sex steroids. There is an urgent need to study both sexes, because they represent the foundation for sex-specific medicine to prevent metabolic and immune diseases. In this context, it is important to note that mitochondria play a crucial role in the regulation of metabolic and immune functions,16,17 and sex differences are known to exist in mitochondrial functions.2,3 Furthermore, current literature suggests that the role of male and female sex steroids in mitochondrial biology is not equal. For example, estrogens, but not androgens, play a crucial role in mitochondrial biology, including mitochondrial biogenesis and respiratory chain complex assembly and function.18–20 In addition, a number of nuclear encoded mitochondrial proteins are located on the X chromosome,21 which may escape X chromosome inactivation (XCI) and contribute to sex differences. Thus, it is possible that mitochondria and mitochondrial proteins carry out sex-dimorphic functions.
New evidence suggests that an evolutionarily conserved pleiotropic protein, prohibitin (PHB, also known as PHB1), has roles in sex differences in adipose and immune functions.22–25 Notably, PHB is known to function as a mitochondrial chaperone26 and an adaptor molecule in plasma membrane signaling [reviewed in Mishra et al.27], but also has multifaceted relationship with sex steroids [reviewed in Mishra et al.28]. In this opinion article, we will provide a viewpoint on the role of mitochondria and PHB in mediating sex-dimorphic adipose and immune functions. Finally, we will conclude that PHB is a potential target for sex-based new therapeutics for metabolic and immune diseases.
Sex differences in adipose tissue distribution and functions
For the same body mass index, women typically have around 10% higher body fat mass than men.29,30 However, fat in women is deposited predominantly in gluteofemoral regions, as opposed to predominantly abdominal accumulation in men. Noticeable sex differences in fat distribution arise during puberty when android and gynoid fat distributions appear for the first time.31–33 Adiposity, especially in the visceral compartment, increases with age in both sexes, and, in women, this redistribution of adipose tissue coincides with the fall in estrogen levels preceding menopause.34,35 The determining role of sex steroids in sex-specific fat distribution has been demonstrated during treatment of transsexual men and women with estradiol (E2) or testosterone.36 However, the cellular and molecular mechanisms by which sex steroids modulate the size of specific fat depots in humans remain largely unknown.
In general, sex differences in adipose tissue distribution relate with whole body metabolism and future health,37,38 and epidemiological and clinical studies have established that gynoid distribution of fat provides resistance to metabolic dysregulation.39,40 Primary factors that may contribute to sex differences in adipose tissue biology and whole body metabolism include adipocyte intrinsic factors and modulatory effects of sex hormones.39,40 In addition, adipose tissue-specific microenvironment may contribute to such differences. For instance, adipose tissue-specific macrophages exhibit sex differences, and are known to play a role in adipocyte functions. Sex differences in biology of adipose tissues would imply that these tissues differ in their ability to perform primary adipocyte functions such as glucose and lipid handling, and adipokines production.41–46 For instance, absence of E2 results in reduced lipid oxidation in women35 and in ovariectomized rodents,47 indicating a role for estrogens in this process. It is interesting to note that higher E2 level, which generally has a positive influence on insulin sensitivity, does not appear to have similar effect during pregnancy. This may be an adaptive change to spare glucose availability for optimal fetal growth.46 It is possible that other pregnancy-related hormones such as progesterone and placental lactogen modulate E2’s role on adipose and immune functions during pregnancy. Thus, the role of sex steroids in adipose and immune functions in pregnant females is not limited to estrogens.
Sex differences in adipose tissue distribution and functions are likely determined by a complex interplay of genetic, hormonal, and epigenetic factors. However, their precise role and relative contribution of regulatory factors and cell autonomous properties remain blurred, and it is largely unknown whether specific fat depots are differentially sensitive to sex steroids, and why adipose tissue in women confers protection against metabolic diseases. In vitro, estrogens stimulate proliferation of human preadipocytes,48,49 whereas androgens inhibit differentiation without affecting proliferation.50,51 Apart from regulating fuel homeostasis, adipose tissue releases a large number of secretory products such as adipokines, chemokines, and cytokines. Some of these factors are known to display sex differences in their secretion and regulation and also contribute to fuel homeostasis.52,53 The sex differences in adipose tissue are not limited to white fat, as females have more active brown adipose tissue than males in both in humans and animal models.54,55
Adipose tissue is also home to different immune cell types, and adipocytes and immune cells interact with each other, which is crucial for their functions. For example, macrophages in adipose tissue play an important role in metabolic homeostasis.56–58 In lean animals, resident macrophages in tissue express genes associated with anti-inflammatory function. Anti-inflammatory (M2) macrophages promote insulin sensitivity by inhibiting pro-inflammatory M1 macrophage activation and associated inflammation. In obese mice with insulin resistance, liver and adipose macrophages are M1-like, and M1 products such as TNF-α are instrumental in causing insulin resistance.57 Like adipocytes, sex differences also exist in various immune cell types including macrophages.59 However, it is not known whether sex differences in adipocytes and macrophages reciprocally affect each other and contribute to sex-specific adipose tissue phenotypes. In addition, it is largely unexplored whether immune components are different in different fat depots and contribute to depot-specific function.
Sex differences in metabolic homeostasis and their implication for obesity and diabetes in males and females have been reviewed recently47,60 and will not be discussed here. In this context, it is important to note that adipose tissue has the ability to aromatize androgens to estrogens and inactivate estrogens through sulfotransferase. It is possible that this attribute can also contribute to sex differences in metabolic function and in immune cells present in adipose tissue. Thus, it is possible that obesity may affect the intracrinology of sex steroids and consequently programming of adipose and immune cells.
PHB
PHB is a ∼30 kDa protein found in multiple cellular compartments including the cell nucleus, mitochondria, and plasma membrane. Its name derived from its original discovery as an anti-proliferative gene. It became subsequently clear that PHB has diverse cellular roles such as regulation of transcription and cell cycle, mitochondrial function, signal transduction in the plasma membrane, anti-oxidation, and anti-inflammation [reviewed in Mishra et al.27]. PHB is evolutionary conserved, being similar in prokaryotes and eukaryotes. PHB is best known as a mitochondrial chaperone, but is also a regulator of transcription, and a participant in signal transduction from the plasma membrane [reviewed in Mishra et al.27 and Ande et al.61]. As a mitochondrial chaperone, PHB stabilizes the mitochondrial genome and proteins related to the respiratory chain, preventing their degradation by proteases. PHB also promotes mitochondrial morphogenesis, and loss of PHB leads to severe changes in mitochondrial membrane potential and morphology, and in destabilization of Optic atrophy 1 protein, which is required for mitochondrial fusion. Conversely, mitochondrial PHB expression increases with cellular injury and protects against oxidative stress [reviewed in Ande et al.61]. Besides roles in cellular processes such as cell proliferation and mitochondrial housekeeping, PHB has fundamental cell type- and tissue-specific functions such as adipogenic and immune functions [reviewed in Mishra et al.28 and Ande et al.61]. The role of PHB in adipogenesis is mediated through its mitochondrial function22,62 (Figure 1), whereas its immune cell-specific functions require membrane-associated cell signaling functions [reviewed in Ande et al.63] (Figure 2).
Figure 1.
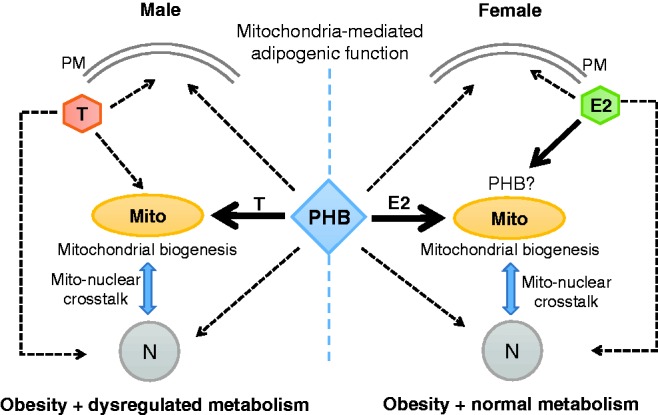
Interaction of PHB with sex steroids in the adipocyte. Estradiol (E2) and testosterone (T) signal to the plasma membrane (PM), mitochondria (Mito), and cell nucleus (N) through their respective receptors located at these cellular compartments. PHB appears to predominantly interact with E2 and T at the Mito level, and allows enhanced mitochondrial biogenesis (Mito-N cross talk) and adipogenesis similarly in both sexes, but their metabolic consequences are sex dimorphic. This would imply that a bi-faceted and sex dimorphic interplay occurs between PHB and sex steroids in adipocytes, i.e. PHB requires sex steroids for adipogenic function and this is modulated by E2 and T differently. PHB-Tg mice and mPHB-Tg mice exhibit sex-specific metabolic phenotypes, but the mice in both sexes are obese, suggesting that mPHB retains its mitochondria mediated adipogenic function. This does not exclude a potential interplay between PHB and sex steroids in the nucleus and plasma membrane. (A color version of this figure is available in the online journal.)
Figure 2.
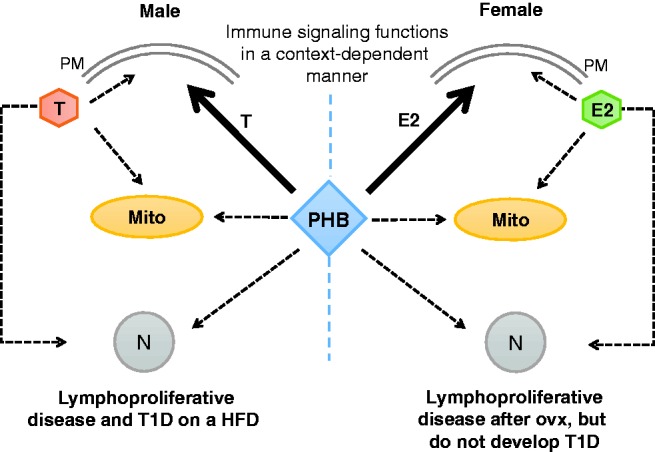
Interaction of PHB with sex steroids in the immune cell. Estradiol (E2) and testosterone (T) signal to the plasma membrane (PM), mitochondria (Mito) and cell nucleus (N) through their respective receptors located at these cellular compartments. PHB appears to be differently modulated by E2 and T at the PM level. Due to PHB phosphorylation at tyrosine-114, male mPHB-Tg mice display lymphoproliferative phenotype and develop T1D on a high-fat diet, whereas female mPHB-Tg mice do not exhibit these abnormalities. Similar to PHB-Tg mice, a potential contribution of the interplay between PHB and sex steroids in other cellular compartments remains a possibility. HFD; high-fat diet; ovx: ovariectomy. (A color version of this figure is available in the online journal.)
PHB-Tg mice display sex differences in adipose phenotype and metabolic dysregulation
To further study the role of PHB in cell compartment-specific adipogenesis and immune functions at the systemic level, we developed transgenic (Tg) mouse models expressing PHB and Y114F-PHB (mPHB) from adipocyte protein-2 (aP2) gene promoter,22,23 which is primarily expressed in adipocytes but also in monocytic macrophages and dendritic cells.64–66 PHB-Tg and mPHB-Tg mice developed obesity in a sex-neutral manner, suggesting that mPHB retains the mitochondria-associated adipogenic function of wild-type PHB23 (Figure 1). However, these mice developed obesity-related metabolic dysregulation such as fatty liver, insulin resistance, and impaired glucose tolerance in a male-specific manner,22–25 revealing a sex-dimorphic role of PHB in adipose tissue and, potentially, in the protective role of estrogen in metabolic and immune dysregulation (Figure 1). Gonadectomy abrogated weight gain, with a greater reduction of subcutaneous than visceral fat in PHB-Tg mice of both sexes, and metabolic dysfunction in male PHB-Tg mice.28,67 With aging, male PHB-Tg developed steatohepatitis and liver tumors, whereas female PHB-Tg mice were protected.24 Further analysis of mitochondrial markers in white adipose tissue from PHB-Tg mice revealed that PHB overexpression in adipocytes enhances mitochondrial biogenesis.22 Sex differences in adipose tissue phenotype was also observed in brown adipose depots, where only male PHB-Tg mice exhibited increased fat accumulation, larger fat droplets, and reduced mitochondrial size.22 Taken together, these findings provided evidence for a critical role of PHB and adipocyte mitochondria in adipose tissue homeostasis, and revealed sex differences in the effect of PHB-induced adipocyte mitochondrial remodeling on whole-body metabolism.22 In addition, these evidences point to an important interconnection between PHB, sex steroids, and mitochondria in mediating sex differences in adipose tissue functions and metabolic regulation (Figure 1). In this context, it should be noted that sex steroid receptors localize to mitochondria and play a role in mitochondrial biology.19,20
Apart from membrane signaling and mitochondrial chaperoning, PHB is also a transcriptional co-regulator, but it is not known whether this nuclear function is involved in adipose and immune regulation. From its major location in the mitochondria, PHB can translocate to the nucleus in response to estrogen,68 and PHB has been associated with the function of mitochondrial transcription factor (Tfam) and nuclear factor-2 (Nrf-2).69,70 Tfam plays an important role in mitochondrial biology, and Nrf-2 regulates transcription of a number of nuclear-encoded mitochondrial proteins.69,70 In addition, PHB and Nrf-2 have been implicated in anti-oxidant defense mechanisms.70 These evidences, along with finding of upregulated mitochondrial biogenesis in white adipose tissue of PHB-Tg mice indicate a potential role of PHB in mitonuclear crosstalk required for mitochondrial biogenesis. An enhanced mitonuclear crosstalk may be the underlying mechanism behind increased mitochondrial biogenesis in white adipose tissue of PHB-Tg mice. In this context, it should be noted that estrogens, which have protective effects against obesity, also play a role in mitochondrial biogenesis.18–20 However, it is not clear whether the role of estrogen in mitochondrial biology is linked to its protective effect against obesity. The PHB-Tg female mice have created an opportunity to dissect the interrelationship among estrogens/estrogen receptor (ER), PHB, and mitochondria in adipose tissue biology and metabolic regulation.
Sex differences in immune health and disease
Sex differences are known to exist in various aspects of immune functions in health and disease. For example, women exhibit a greater immune response to vaccination and are more susceptible to autoimmune diseases than men, whereas men are more susceptible to infectious diseases and cancer.71,72 A meta-analysis of epidemiological studies on infectious diseases found a higher incidence of infectious diseases in adult men.73 This male bias in susceptibility to pathogens has also been reported in rodents.12 Even though substantial sex differences occur in immune health and disease in humans and rodent models, our understanding of the underlying mechanisms remains poor. Likely factors involve sex chromosomes-linked genes, epigenetic modifications, sex steroid hormones, and environmental factors.74,75
Recently, Furman et al.1 found an association of genes that are upregulated by testosterone and are involved in lipid metabolism, with poor antibody-neutralizing response to influenza vaccine, indicating a link between testosterone regulated genes and response to vaccination. Furthermore, these authors proposed that testosterone directly regulates transcriptional factors involved in immune activation, which may play a role in the regulation of lipid metabolism by testosterone in immune cells. Of note, emerging evidence suggests that metabolic changes within immune cells (immunometabolism) play a major role in their functional polarization76 and could be important drivers of the differences in immune responses between males and females.
Besides testosterone, there is also evidence that estrogens have a direct effect on immune cells. For example, E2 positively regulates the toll-like receptor (TLR)-mediated response of plasmacytoid dendritic cells in vivo through cell-intrinsic ERα signaling.77 An ERα-mediated effect of E2 has also been reported on NF-κB p65 transcriptional activity and levels of marker genes in murine macrophages.78 Similarly, a direct effect of E2 has been reported on enhancement of primary antigen-specific CD2 T cell expansion and Th1 development.79 Such differences in immune cell responses to sex steroids are important because of their role in innate and adaptive immunity, and may contribute to sex differences in immune health and disease.
The possibility that sex steroids affect T-cell function led us to query their influence on lymphoma. It is known that women have a lower incidence and a better prognosis of B- and T-cell lymphomas than men,80,81 and several strong hints point at a protective function of estrogens in lymphomagenesis.82 Lymphoma cells primarily express ERβ, and this receptor, in contrast to ERα, seems to mostly act anti-proliferative and pro-apoptotic through the execution of a specific and complex gene program, a hallmark of many tumor-suppressive transcriptional regulators.82 Recently, Yakimchuk et al.83 provided convincing evidence that activation of ERβ in lymphoma acts tumor suppressive, through modulation of genes that cause a reduction in proliferation and survival of tumor cells as well as a reduction of tumor vascularization and dissemination.
These findings indicate that estrogen and androgen both exert important effects on the immune system, and may act through multiple cell types. It is likely that sensitivity to sex hormones may vary widely between different immune cell types. Although there is accumulating observational data that describe the importance of the effects of sex hormones on immune cells and their function, there is a paucity of mechanistic data, which would inform/translate these observations into clinical practice.
PHB-Tg and mPHB-Tg mice display sex differences in immune phenotype
The mPHB-Tg mice expressing Y114F-PHB in monocytic macrophages and dendritic cells display male-specific immunosuppressive phenotype and develop lymph node tumors, whereas female mPHB-Tg mice are protected from tumor development, suggesting a potential role of sex steroids in lymphomatosis23 (Figure 2). As speculated, ovariectomy in female mPHB-Tg mice caused metabolic dysregulation and tumor development similar to their male counterparts, confirming a protective role of estrogens.23 Intriguingly, on a high-fat diet, male mPHB-Tg mice developed type 1 diabetes (T1D) instead of tumors.25 The development of tumors and T1D in a mutually exclusive manner would imply that PHB has a role in immune checkpoints that play crucial role in self-tolerance and tumor surveillance, and that this regulatory role is linked to the phosphorylation of PHB at tyrosine-114. Thus, a protective role of testosterone against autoimmune disease, as discussed above, was not observed in male mPHB-Tg mice on high-fat diet. Because PHB is known to co-repress androgen action,84 it is possible that this attribute along with metabolic stress, in the form of high-fat diet, may have contributed to the development of T1D in mPHB-Tg mice in a male-specific manner. Identification of PHB in integrating immunomodulatory effects of sex steroids is a step forward in this direction. This evidence also supports a role for PHB in reciprocal effects of sex differences in metabolic and immune functions through its modulatory effect on sex steroid action in adipocytes and macrophages/dendritic cells. It would be interesting to study the role of PHB in modulating sex steroid actions in different immune cell types.
Sex hormones exert potent effects on immune cell subsets by binding to nuclear receptors that act as ligand-dependent transcription factors,85 to plasma membrane G-protein coupled receptors that initiate signal transduction pathways,86,87 and to mitochondrial steroid receptors.88,89 In this context, it is important to note that PHB appears to modulate all three modes of action of sex steroids (Figure 2). Moreover, sex steroids also appear to modulate PHB functions in different cellular compartments, as gonadectomy in PHB-Tg and m-PHB-Tg mice alters their metabolic and immune phenotypes.23,67,90 These evidences suggest a multifaceted relationship between PHB and sex steroids in the regulation of metabolic and immune functions, which may work in a context-dependent manner.
Do mitochondria have a role in sex-dimorphic functions?
The ooplasm has the unique ability to distinguish sperm mitochondria from self-mitochondria, for selective degradation of paternal mitochondria to ensure maternal mitochondria inheritance. This would imply that sex differences exist in mitochondria that must be acquired at some point in males’ life from birth to puberty, or at the time of fertilization. This is because of the maternal inheritance of mitochondria. This fundamental aspect of mitochondrial biology raises two interrelated questions about the acquisition of sex differences and its potential role in mediating sex differences in different cells and tissues. We propose that mitochondria and mitochondrial proteins play a crucial role in mediating sex differences in cellular functions.
This is because fundamental aspects of mitochondrial biology, including the anaplerotic citric acid cycle (Krebs cycle) and energy production are adapted to serve cell type or tissue-specific functions. For instance, ATP production is linked to insulin secretion in pancreatic β-cells, whereas it is associated with lipogenesis in adipocytes.91 Some of these cell- or tissue-specific functions display sex differences,46 which raises an important question about the potential role of mitochondria in sex differences. A number of potential mechanisms exist that could be involved in the role of mitochondria in sex differences. First, sex steroids regulate mitochondrial functions, and sex differences have been described in mitochondrial biogenesis in white adipose tissue.92 Mitochondrial biogenesis plays a central role in adipogenesis, which is higher in females than males, and estrogens are known to play a role in mitochondrial biogenesis.18–20 Moreover, most of the adipose tissue and liver-related alterations in diseases occur in parallel with an impairment of mitochondrial function.93–95 It has also been found that brown adipose tissue, liver, skeletal muscle, brain, and heart exhibit more differentiated and functional mitochondria in female rats than in males,55,96–99 and this is accompanied by a better insulin sensitivity profile100 and a greater brown adipose tissue thermogenesis.101 Interestingly, sex differences in brown adipose tissue structure, including mitochondria, are further amplified in PHB-Tg mice, where a number of mitochondrial markers are upregulated in adipose tissue.22 This dimorphism is likely to be mediated by estrogens, as they regulate and favor mitochondrial function and biogenesis.102 Moreover, the presence of ERα and ERβ in mitochondria and white adipose tissue19,103 further supports the idea of a possible involvement of estrogens in sex-related differences in mitochondrial function. Second, the role of mitochondria in sex differences in health and disease may involve epigenetic mechanisms, because mitochondrial DNA (mtDNA) copy numbers have been reported to play a role in epigenetic changes in the nuclear genome.104 Finally, genes located on X chromosome may escape its inactivation and contribute to sex dimorphism in mitochondrial functions. Recently, Balaton et al.105 have catalogued consensus inactivation status of X-linked genes from genome-wide studies. We searched the mitochondrial proteins database MitoCarta106 and found more than 25 mitochondrial proteins encoding genes located on X chromosome in humans (Table 1). In mice, these genes are also located on X chromosome, except SLC25A6. All these X-linked genes encoding mitochondrial proteins are either escape from, subject to, or variably escape from XCI (Table 1). Similarly, we searched XCI consensus dataset105 for inactivation status of immune-related genes on X chromosome and identified a number of genes (Table 2). Therefore, escape from XCI might contribute to sex differences in cellular functions involved in metabolic and immune functions, because of crucial role of mitochondria in major metabolic tissues and in the functional plasticity of immune cells.76 Moreover, polymorphism in genes on the X chromosome that encode for immunological proteins may lead to sex differences in immune response. For example, TLR-7, located on the X chromosome, can escape X inactivation, resulting in higher expression in females than males.107
Table 1.
Mitochondrial protein encoding genes located on X chromosome in humans and their consensus call for escape from X chromosome inactivation (XCI).
| Gene ID | Gene name | Consensus call | Encoded proteins and their known/potential role |
|---|---|---|---|
| 212 | ALAS2 | Subject to | Catalyzes the first step in the heme biosynthetic pathway. Defects in this gene cause X-linked pyridoxine-responsive sideroblastic anemia. Restricted expression in bone marrow. |
| 2710 | GK | Subject to | A key enzyme in the regulation of glycerol metabolism. It catalyzes the phosphorylation of glycerol by ATP, yielding ADP and glycerol-3-phosphate. |
| 3052 | HCCS | Subject to | Encodes an enzyme that covalently links a heme group to the apoprotein of cytochrome c. Defects in this gene are a cause of microphthalmia syndromic type 7. |
| 4128 | MAOA | Mostly subject to | Catalyzes the oxidative deamination of amines, such as dopamine, norepinephrine, and serotonin. Mutation of this gene results in Brunner syndrome. |
| 4515 | MTCP1 | Subject to | Involvement in some t(X;14) translocations associated with mature T-cell proliferations. May be involved in leukemogenesis. |
| 5009 | OTC | Subject to | A mitochondrial matrix enzyme. Missense, nonsense, and frameshift mutations in this enzyme lead to ornithine transcarbamylase deficiency, which causes hyperammonemia. |
| 9016 | SLC25A14 | Subject to | A member of the larger family of mitochondrial anion carrier proteins (MACP). |
| 10245 | TIMM17B | Mostly subject to | A multipass transmembrane protein that forms an integral component of the mitochondrial translocase TIM23 complex. This complex facilitates the transport of mitochondrial proteins from the cytosol across the mitochondrial inner membrane and into the mitochondrion |
| 9131 | AIFM1 | Subject to | A flavoprotein essential for nuclear disassembly in apoptotic cells, and found in the mitochondrial intermembrane space in healthy cells. Induction of apoptosis results in the translocation of this protein to the nucleus where it affects chromosome condensation and fragmentation. Also induces mitochondria to release the apoptogenic proteins cytochrome c and caspase-9. |
| 4694 | NDUFA1 | Subject to | An essential component of complex I of the respiratory chain, which transfers electrons from NADH to ubiquinone. |
| 11258 | CA5B | Escape from | Catalyzes the reversible hydration of carbon dioxide and participates in a variety of biological processes, including respiration, calcification, acid-base balance, bone resorption, and formation of aqueous humor, cerebrospinal fluid, saliva, and gastric acid. |
| 1349 | COX7B | Variable escape | Catalyzes electron transfer from reduced cytochrome c to oxygen. |
| 65991 | FUNDC2 | Subject to | FUN14 domain containing 2 protein, ubiquitously expressed in heart and ovary. |
| 4129 | MAOB | Subject to | An enzyme located in the mitochondrial outer membrane. It catalyzes the oxidative deamination of biogenic and xenobiotic amines and plays an important role in the metabolism of neuroactive and vasoactive amines in the central nervous system and peripheral tissues. This protein preferentially degrades benzylamine and phenylethylamine. |
| 5160 | PDHA1 | Subject to | Catalyzes the conversion of pyruvate to acetyl-CoA and CO(2), and provides the primary link between glycolysis and the tricarboxylic acid cycle. |
| 3028 | HSD17B10 | Mostly subject to | Encodes 3-hydroxyacyl-CoA dehydrogenase type II, a member of the short-chain dehydrogenase/reductase superfamily. A mitochondrial protein that catalyzes the oxidation of a wide variety of fatty acids and steroids, and is a subunit of mitochondrial ribonuclease P, which is involved in tRNA maturation. The protein has been implicated in the development of Alzheimer disease. |
| 1678 | TIMM8A | Mostly subject to | Involved in the import and insertion of hydrophobic membrane proteins from the cytoplasm into the mitochondrial inner membrane. The gene is mutated in Mohr-Tranebjaerg syndrome/Deafness Dystonia Syndrome (MTS/DDS) and it is postulated that MTS/DDS is a mitochondrial disease caused by a defective mitochondrial protein import system. |
| 292 | SLC25A5 | Subject to | Functions as a gated pore that translocates ADP from the cytoplasm into the mitochondrial matrix and ATP from the mitochondrial matrix into the cytoplasm. |
| 54539 | NDUFB11 | Subject to | A subunit of the multisubunit NADH:ubiquinone oxidoreductase (complex I) and has NADH dehydrogenase activity and oxidoreductase activity. It transfers electrons from NADH to ubiquinone. |
| 2182 | ACSL4 | Subject to | An isozyme of the long-chain fatty-acid-coenzyme A ligase family, which preferentially utilizes arachidonate as substrate; its absence may contribute to the cognitive disability or Alport syndrome. |
| 203427 | SLC25A43 | Mostly subject to | A member of the mitochondrial carrier family of proteins. |
| 293 | SLC25A6 | PAR (Escape) | A member of the mitochondrial carrier subfamily of solute carrier protein genes. The product of this gene functions as a gated pore that translocates ADP from the cytoplasm into the mitochondrial matrix and ATP from the mitochondrial matrix into the cytoplasm. The protein is implicated in the function of the permeability transition pore complex, which regulates the release of mitochondrial products that induce apoptosis. |
| 56474 | CTPS2 | Mostly escape | Catalyzes the formation of CTP from UTP with the concomitant deamination of glutamine to glutamate. This protein is the rate-limiting enzyme in the synthesis of cytosine nucleotides, which play an important role in various metabolic processes and provide the precursors necessary for the synthesis of RNA and DNA. |
| 5165 | PDK3 | Subject to | A member of multienzyme complex that catalyzes the overall conversion of pyruvate to acetyl-CoA and CO2. It provides the primary link between glycolysis and the tricarboxylic acid cycle, and thus is one of the major enzymes responsible for the regulation of glucose metabolism. |
| 3421 | IDH3G | Subject to | Catalyzes the oxidative decarboxylation of isocitrate to 2-oxoglutarate. |
| 27301 | APEX2 | Subject to | Shown to have a weak class II apurinic/ apyrimidinic endonuclease activity. May play an important role in both nuclear and mitochondrial base excision repair. |
| 23597 | ACOT9 | Subject to | A mitochondrial acyl-CoA thioesterase of unknown function. Ubiquitously expressed in heart. |
| 215 | ABCD1 | Mostly subject to | A member of the superfamily of ATP-binding cassette transporters, which is involved in peroxisomal import of fatty acids/fatty acyl-CoAs. Defects in this gene have been identified as the underlying cause of adrenoleukodystrophy, an X-linked demyelinating disorder. |
PAR: pseudoautosomal regions.
Table 2.
Immune-related genes located on X chromosome in humans and their consensus call for escape from XCI.
| Gene ID | Gene names | Consensus call | Encoded proteins and their known or potential importance in immune functions |
|---|---|---|---|
| 64109 | CRLF2 | PAR | Receptor for stromal lymphopoietin. Together with interleukin 7 receptor, activates STAT3, STAT5, and JAK2 pathways. |
| 1438 | CSF2RA | PAR | The alpha subunit of the heterodimeric receptor for CSF2, a cytokine which controls the production, differentiation, and function of granulocytes and macrophages. |
| 3563 | IL3RA | PAR (Escape) | The alpha subunit of heterodimeric IL3, CSF2/GM-CSF, 3 and IL5. |
| 286530 | P2RY8 | PAR | Belongs to the family of G-protein coupled receptors, preferentially activated by adenosine and uridine nucleotides. Broad expression in lymph node and spleen. |
| 8227 | AKAP17A | PAR (Escape) | A protein kinase A anchoring protein. A part of spliceosome complex, ubiquitous expression in spleen and bone marrow. |
| 401577 | CD99P1 | PAR (Escape) | CD99 molecule pseudogene 1, ubiquitous expression in bone marrow. |
| 4267 | CD99 | PAR | A cell surface glycoprotein involved in leukocyte migration, T-cell adhesion, ganglioside GM1 and transmembrane protein transport, and T-cell death by a caspase-independent pathway. |
| 51284 | TLR7 | Subject to | A fundamental role in pathogen recognition and activation of innate immunity. |
| 51311 | TLR8 | No call | A fundamental role in pathogen recognition and activation of innate immunity. |
| 7114 | TMSB4X | No call | Role in cell proliferation, migration, and differentiation. Broad expression in spleen and lymph node. |
| 5277 | PIGA | Subject to | Required for synthesis of N-acetylglucosaminyl phosphatidylinositol (GlcNAc-PI), the first intermediate in the biosynthetic pathway of GPI anchor, a glycolipid found on many blood cells and which serves to anchor proteins to the cell surface. |
| 8233 | ZRSR2 | Escape from | An essential splicing factor, and may play a role in network interactions during spliceosome assembly. Ubiquitous expression in lymph node and spleen. |
| 369 | ARAF | Subject to | Belongs to the RAF subfamily of Ser/Thr protein kinase family and may be involved in cell growth and development. Ubiquitous expression in bone marrow and spleen. |
| 5199 | CFP | Subject to | A plasma glycoprotein that positively regulates the alternative complement pathway of the innate immune system. Biased expression in spleen and bone marrow. |
| 7454 | WAS | Variable escape | Expressed exclusively in hematopoietic cells and is involved in transduction of signals from receptors on the cell surface to the actin cytoskeleton. |
| 50943 | FOXP3 | Subject to | A member of the forkhead/winged-helix family of transcriptional regulators. Defects in this gene are the cause of immunodeficiency polyendocrinopathy and X-linked autoimmunity-autoimmune deficiency syndrome. |
| 11326 | VSIG4 | No call | A receptor for the complement component 3 fragments C3b and iC3b and may be a negative regulator of T-cell responses. |
| 3476 | IGBP1 | Mostly subject to | Proliferation and differentiation of B cells. |
| 3561 | IL2RG | No call | An important signaling component of many IL receptors. Mutations in this gene cause X-linked severe combined immunodeficiency (XSCID), as well as X-linked combined immunodeficiency (XCID). |
| 8473 | OGT | Mostly subject to | A glycosyltransferase that catalyzes the addition of a single N-acetylglucosamine in O-glycosidic linkage to serine or threonine residues. Ubiquitous expression in lymph node and spleen. |
| 2833 | CXCR3 | Subject to | A G protein-coupled receptor with selectivity for three chemokines, termed CXCL9/Mig, CXCL10/IP10 and CXCL11/I-TAC. Binding of chemokines to this protein induces cellular responses that are involved in leukocyte traffic, most notably integrin activation, cytoskeletal changes and chemotactic migration. |
| 695 | BTK | Subject to | A crucial role in B-cell development. Mutations in this gene cause X-linked agammaglobulinemia type 1, which is an immunodeficiency characterized by the failure to produce mature B lymphocytes, and associated with a failure of Ig heavy chain rearrangement. |
| 3597 | IL13RA1 | Mostly subject to | Serves as a primary IL13-binding subunit of the IL13 receptor, and may also be a component of IL4 receptors. Binds to TYK2, which lead to the activation JAk1, STST3 and STAT6 induced by IL13 and IL4. |
| 4068 | SH2D1A | Variable escape | Plays a major role in the bidirectional stimulation of T and B cells mediated through SH2 domain. |
| 54440 | SASH3 | Subject to | May function as a signaling adapter protein in lymphocytes mediated through SH3 domain. Biased expression in lymph node and spleen. |
| 2000 | ELF4 | Mostly subject to | A transcriptional activator involved in natural killer cell development and function, innate immunity, and induction of cell cycle arrest in naive CD8+ cells. |
| 959 | CD40LG | Variable escape | Expressed on the surface of T cells and regulates B cell function by engaging CD40 on the B cell surface. |
| 139135 | PASD1 | No call | A cancer-associated antigen that can stimulate autologous T-cell responses. Considered to be a potential immunotherapeutic target for the treatment of various hematopoietic malignancies, including diffuse large B-cell lymphoma. |
| 393 | ARHGAP4 | Discordant | A role in the regulation of small GTP-binding proteins belonging to the RAS superfamily. Biased expression in spleen and bone marrow. |
| 3654 | IRAK1 | Mostly subject to | IL-1 receptor-associated kinase 1 partially responsible for IL1-induced upregulation of the transcription factor NF-kappa B. |
| 8517 | IKBKG | Mostly subject to | The regulatory subunit of the inhibitor of kappaB kinase (IKK) complex, which activates NF-kappaB resulting in activation of genes involved in inflammation, immunity, cell survival, and other pathways. |
| 139716 | GAB3 | Subject to | Functions as scaffolding/docking proteins and involved in several growth factor and cytokine signaling pathways. Binds SHP2 tyrosine phosphatase and GRB2 adapter protein and facilitates macrophage differentiation. |
| 100272147 | MTCP1NB | No call | Involvement in some t(X;14) translocations associated with mature T-cell proliferations. |
| 4515 | MTCP1 | Subject to | Involvement in some t(X;14) translocations associated with mature T-cell proliferations. May be involved in leukemogenesis. |
| 3581 | IL9R | Subject to | Mediates the biological effects of IL9. The ligand binding of this receptor leads to the activation of various JAK kinases and STAT proteins. |
PAR: pseudoautosomal regions.
Sex steroid hormones in sex differences in adipose and immune functions
The two major sex steroid hormones, E2 and testosterone, although derived from the same precursor, cholesterol, belong to two different sub-groups of steroid hormone family members, namely estrogens and androgens, which work through different receptors and have different target genes. Thus, differences inherent to the structure and action of male and female sex steroid hormones expectedly may result in sex differences. This is because sex steroids work on almost all cell and tissue types in the body and have wide ranging roles in various physiological processes. Thus, it would not be a surprise if sex differences of different magnitude exist in almost all cell and tissue types. In this context, it is important to note that these pleiotropic effects of estrogens are mediated through two main receptor isoforms, ERα and ERβ, which display different expression pattern in various cells and tissue types108 and have overlapping as well as distinct target genes.109 This is not the case with testosterone, which works through a single receptor. Thus, the differential expression of sex steroid receptors in various cell and tissue types can also contribute to sex differences in physiology and pathophysiology.
Estrogens play a crucial role in suppressing the development of chronic inflammatory diseases110 and in mitigating the impact of inflammation that occurs in diseases such as obesity and type 2 diabetes.111 Existing evidence indicates that adipose tissue becomes inflamed in obesity, which highly correlates with obesity-related insulin resistance, other metabolic impairments,112,113 and cancer development.114 Thus, preventing or treating adipose tissue inflammation is important to prevent or attenuate the development of metabolic diseases. Notably, there appears to be sex differences in the prevalence of diseases such as cancer, obesity, type 2 diabetes, and cardiovascular disease, with men presenting higher prevalence and disease risk compared to women.115 However, after menopause, women become as susceptible as men to obesity-related diseases.111 This is associated with increased production of inflammatory cytokines such as TNF-α and IL-6 due to loss of estrogens during menopausal transition.116 ERα appears to be the predominant ER in adipose tissue,115,117 and ERα polymorphism has been linked to adiposity, insulin resistance, and inflammation.118 ERα knockdown in 3T3-L1 adipocytes leads to increased level of inflammatory markers.115 Furthermore, E2 has been shown to work in a non-genomic manner and inhibit inflammation by modulating NF-κB signaling through activation of PI3K-Akt pathway.119 Thus, the possibility of similar action of E2 on immune cells exists. In addition, intrinsic differences in cellular response may also exist in cells from male and female. There is a need to better define how these hormones function at the cellular level to reduce inflammation, because there is increasing interest in attaining clinical benefits of estrogens while avoiding side effects. In addition to ER, females possess an extra sex steroid hormone, progesterone, produced after ovulation and during pregnancy, which modulates the action of ER and has immunomodulatory and developmental roles120; however, because of its coexistence with E2 in premenopausal women, independent effects of progesterone on metabolic and immune functions are difficult to dissect out.
Testosterone is an important determinant of body composition in men, promoting growth of lean mass and suppressing deposition of fat.121 Thus, testosterone deficiency is expected to have opposite effects on adipose tissue. Epidemiological studies have found a relationship between low testosterone and obesity in men122 and support the notion that testosterone deficiency in men leads to the development of metabolic syndrome and its related increase in cardiovascular disease risk.123 Some of these evidences came from prostate cancer patients who are on androgen deprivation therapy (ADT). Men undergoing long-term ADT show significant insulin resistance and hyperglycemia compared with non-ADT and control groups.124,125 Furthermore, there is growing evidence suggesting a beneficial effect of testosterone on body composition to reduce visceral obesity and related metabolic syndrome.126,127 The underlying mechanisms by which androgen/androgen receptor (AR) signaling regulates metabolic homeostasis in men are complex and likely to involve crosstalk with insulin target tissues. Evidence derived from various AR knockout mouse models revealed tissue-specific AR signaling that is involved in the regulation of metabolism.123 A better understanding of the role of androgens on metabolism is of substantial clinical importance, because androgen-deprivation is a standard first-line treatment and fundamental management for men with advanced prostate cancer and ADT-related metabolic syndrome may lead to earlier development of castration-resistant prostate cancer.
E2-ER in mitochondria and their potential association with PHB
A number of studies have demonstrated that functional ERα and ERβ are present in mitochondria where they play a role in mitochondrial biology. For example, mitochondrial ERs have been implicated in E2-induced mitochondrial transcription,19 and it has been shown that the D-loop of mtDNA contains putative estrogen response elements.128,129 ERs mitochondrial target genes include those encoding proteins of the respiratory chain complex (MRC).19 MRC proteins also include many nuclear encoded proteins, and mitochondrial genome is known to work in coordination with nuclear genome. Therefore, it is likely that nuclear and mitochondrial ERs also work in a coordinated manner, and that PHB may modulate ER function in both compartments. Moreover, E2 leads to activation of ERK1/2 (via MAPK) and Bad (via PKA),19 and both signaling molecules are modulated by PHB [reviewed in Mishra et al.27 and Ande et al.27,63]. PHB also modulates STAT3 signaling, which has been implicated in mitochondrial biology,130 and interacts with Tfam and thus regulates mtDNA copy number, as Tfam is a DNA-encoded, mitochondrial protein that is crucial for replication, transcription, and maintenance of mtDNA.131,132 Thus, a possibility exists that PHB may modulate the different effects of estrogen on mitochondria at several levels (signaling, transcription, mtDNA copy number) and contribute to sex differences in cellular functions (Figures 1 and 2). Whether androgens have a similar role in mitochondrial biology remains unclear.
Epigenetic characteristics and sex differences
A complete knowledge of the sequence of genomic DNA of an individual is insufficient to predict the gene expression pattern and metabolism found in somatic cells, because of the crucial regulatory role of epigenetic factors.133 Several types of epigenetic characteristics are known that distinguish somatic cells. Two of these are nuclear DNA methylation and mtDNA copy number.133 Over 150 tissue-specific DNA methylation sites have been identified,134 and the mitochondrial DNA copy number varies widely from a few hundreds to thousands.135 Recently, Smiraglia et al.104 have shown that mtDNA copy number changes have a profound effect on the methylation pattern of nuclear genes. Although this discovery was made in the context of tumorigenesis, a role for such a regulatory mechanism in sex differences associated with other conditions remains a possibility. For example, E2 action on mtDNA and mitochondrial biogenesis may lead to metabolic and immune protection. Similarly, sex differences in mitochondrial phenotype observed in PHB-Tg mice may have contributed to sex differences in their metabolic and immune phenotypes. In this context, it should be noted that PHB regulates mitochondrial transcription factor Tfam as well as nuclear transcription factor Nrf2, which play an important role in the regulation of a number of mitochondrial DNA encoded proteins and nuclear encoded mitochondrial proteins, respectively. Thus, PHB may play a role in the regulation epigenetic influence of mitochondrial attributes in sex-dimorphic functions.
Escape from XCI and its potential role in sex differences
In mammals, sex is chromosomally determined with the presence or absence of Y chromosome generally resulting in XY male and XX female.105 This genetic difference leads to sex-specific gonad development and consequently sex- and gonad-specific hormone production. Thus, the major contributing factors in sexual dimorphism in mammals include expression of sex-linked genes and differential hormonal regulation of some gene pathways and functions by male and female sex steroid hormones.105 The sex difference in the expression of most X-linked genes is minimized by XCI through epigenetic mechanisms. However, there are exceptions, because some genes could escape X chromosome silencing at variable frequencies.136 Furthermore, XCI plays a role in achieving dosage compensation of genes located on pseudoautosomal regions of X chromosomes, which have homologous genes on the Y counterpart.137 Escape from XCI can lead to male–female expression differences, particularly in humans.138 Gencode currently lists 1144 genes on the human X chromosome,139,140 and approximately 15% of X-linked genes consistently escape from this inactivation and another 15% of genes vary between individuals or tissues in whether they are subject to, or escape from, inactivation.105 The X chromosome contains a maximum number of immune-related genes and many nuclear encoded mitochondrial genes, and it is possible that escape from XCI affects immune response and is responsible for different immunological responses between sexes and individual females.
The list of XCI contains not only protein-coding genes but also microRNA (miRNA) and noncoding RNA. It has been recently reported that the human X chromosome has a higher density of miRNA (10% of the approximate 800 miRNA in the human genome) when compared with autosomes, whereas the Y chromosome has no miRNA.141 It is possible that XCI may affect X-linked miRNA, immune genes, and mitochondrial protein encoding genes. Differential expression of some of these X-linked genes may predispose to autoimmunity. Examples are CD40LG, CD70, and CD11, which are overexpressed in B and T cells in women with systemic lupus erythematosus.142–144 Thus, the potential contribution of XCI on sex differences in health and disease in general and in metabolic and immune functions in particular is substantial.
PHB as a potential target for sex-based new therapeutics
As discussed above, the major intrinsic factors that contribute to sex differences are sex chromosomes, sex steroid hormones, epigenetics, and escape from XCI.105 However, this aspect of human biology has not been capitalized yet for sex-based precision medicine. The discovery of PHB as mediating sex differences in metabolic and immune function has opened a new research direction in this field. It appears that there are multiple mechanisms by which PHB can contribute to sex differences in metabolic and immune functions, and these may include modulation of genomics, epigenetics, and membrane signaling functions of sex steroids as well as mitochondrial functions (Figure 3). As pleiotropic actions of PHB are mediated in a cell compartment- and tissue-specific manner, it might be possible to selectively target PHB for therapeutic purposes in a cell type- and sex-specific manner. In addition to brown adipose tissue, white adipose tissue, macrophages and dendritic cells, PHB plays a role in mast cells, B and T cells.145–147 However, it is not known whether PHB has similar sex-dimorphic role in these cell types. Furthermore, many of these functions of PHB are also shared by its homologous protein PHB2 [reviewed in Ande et al.61]. It is possible that PHB2 may have sex-dimorphic functions similar to PHB, which is not known and requires further investigation. For example, PHB2 deficiency in mouse forebrain has been reported to cause neurodegenerative disease,148 which affects men and women differently. Whether PHB2 contributes to sex differences in neurodegenerative disease, however, is not known. Apart from shared attributes, PHB and PHB2 appear to have protein-specific functions.61 Thus, PHB may serve as a unique cell- and tissue-specific therapeutic target for metabolic and immune diseases in a sex-specific manner. Getting a more complete understanding of the relationship between PHB and sex steroid actions in different cellular compartments and cell types will lead to new insights into the underlying mechanisms. Targeting these pathways would characterize a fresh conceptual approach that will contribute to innovative regimens for sex-specific prevention and treatment of a wide variety of medical conditions.1
Figure 3.
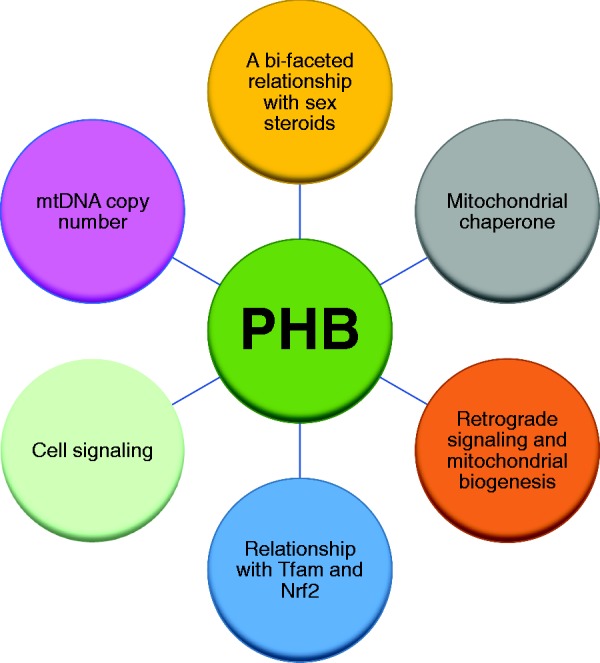
A schematic showing known functions of PHB that may potentially contribute to sex differences in mitochondrial, adipose, and immune functions. (A color version of this figure is available in the online journal.)
Authors’ contributions
SM and BLGN wrote the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared that there is no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
SM is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC, RGPIN-2017–04962), Research Manitoba and Health Sciences Centre Foundation.
References
- 1.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiébaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A 2014; 111:869–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalifa AR, Abdel-Rahman EA, Mahmoud AM, Ali MH, Noureldin M, Saber SH, Mohsen M, Ali SS. Sex-specific differences in mitochondria biogenesis, morphology, respiratory function, and ROS homeostasis in young mouse heart and brain. Physiol Rep 2017; 5:e13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ventura-Clapier R, Moulin M, Piquereau J, Lemaire C, Mericskay M, Veksler V, Garnier A. Mitochondria: a central target for sex differences in pathologies. Clin Sci 2017; 131:803–22 [DOI] [PubMed] [Google Scholar]
- 4.Marini S, Morotti A, Ayres AM, Crawford K, Kourkoulis CE, Lena UK, Gurol EM, Viswanathan A, Goldstein JN, Greenberg SM, Biffi A, Rosand J, Anderson CD. Sex differences in intracerebral hemorrhage expansion and mortality. J Neurol Sci 2017; 379:112–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alabas OA, Gale CP, Hall M, Rutherford MJ, Szummer K, Lawesson SS, Alfredsson J, Lindahl B, Jernberg T. Sex differences in treatments, relative survival, and excess mortality following acute myocardial infarction: national cohort study using the SWEDEHEART registry. J Am Heart Assoc 2017; 6:e007123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zissimopoulos JM, Barthold D, Brinton RD, Joyce G. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol 2017; 74:225–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrow EH. The evolution of sex differences in disease. Biol Sex Differ 2015; 6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold AP. Promoting the understanding of sex differences to enhance equity and excellence in biomedical science. Biol Sex Differ 2010; 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tannenbaum C, Schwartz JM, Clayton JA, de Vries G, Sullivan C. Evaluating sex as a biological variable in preclinical research: the devil in the details. Biol Sex Differ 2016; 7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillaume M, Handgraaf S, Fabre A, Raymond-Letron I, Riant E, Montagner A, Vinel A, Buscato M, Smirnova N, Fontaine C, Guillou H, Arnal JF, Gourdy P. Selective activation of estrogen receptor α activation function-1 is sufficient to prevent obesity, steatosis, and insulin resistance in mouse. Am J Pathol 2017; 187:1273–87 [DOI] [PubMed] [Google Scholar]
- 11.Ross R, Shaw KD, Rissanen J, Martel Y, de Guise J, Avruch L. Sex differences in lean and adipose tissue distribution by magnetic resonance imaging: anthropometric relationships. Am J Clin Nutr 1994; 59:1277–85 [DOI] [PubMed] [Google Scholar]
- 12.Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev 2000; 24:627–38 [DOI] [PubMed] [Google Scholar]
- 13.Klein SL, Poland GA. Personalized vaccinology: one size and dose might not fit both sexes. Vaccine 2013; 31:2599–600 [DOI] [PubMed] [Google Scholar]
- 14.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013; 339:1084–8 [DOI] [PubMed] [Google Scholar]
- 15.Davis MM. A prescription for human immunology. Immunity 2008; 29:835–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boudina S, Graham TE. Mitochondrial function/dysfunction in white adipose tissue. Exp Physiol 2014; 99:1168–78 [DOI] [PubMed] [Google Scholar]
- 17.Walker MA, Volpi S, Sims KB, Walter JE, Traggiai E. Powering the immune system: mitochondria in immune function and deficiency. J Immunol Res 2014; 2014:164309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galmés-Pascual BM, Nadal-Casellas A, Bauza-Thorbrügge M, Sbert-Roig M, García-Palmer FJ, Proenza AM, Gianotti M, Lladó I. 17β-estradiol improves hepatic mitochondrial biogenesis and function through PGC1B. J Endocrinol 2017; 232:297–308 [DOI] [PubMed] [Google Scholar]
- 19.Chen JQ, Cammarata PR, Baines CP, Yager JD. Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim Biophys Acta 2009; 1793:1540–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao J, Zhao L, Mao Z, Chen S, Wong KC, To J, Brinton RD. Potentiation of brain mitochondrial function by S-equol and R/S-equol estrogen receptor β-selective phytoSERM treatments. Brain Res 2013; 1514:128–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichtenstein DA, Crispin AW, Sendamarai AK, Campagna DR, Schmitz-Abe K, Sousa CM, Kafina MD, Schmidt PJ, Niemeyer CM, Porter J, May A, Patnaik MM, Heeney MM, Kimmelman A, Bottomley SS, Paw BH, Markianos K, Fleming MD. A recurring mutation in the respiratory complex 1 protein NDUFB11 is responsible for a novel form of X-linked sideroblastic anemia. Blood 2016; 128:1913–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ande SR, Nguyen KH, Padilla-Meier GP, Wahida W, Nyomba BL, Mishra S. Prohibitin overexpression in adipocytes induces mitochondrial biogenesis, leads to obesity development, and affects glucose homeostasis in a sex-specific manner. Diabetes 2014; 63:3734–41 [DOI] [PubMed] [Google Scholar]
- 23.Ande SR, Nguyen KH, Padilla-Meier GP, Nyomba BL, Mishra S. Expression of a mutant prohibitin from the aP2 gene promoter leads to obesity-linked tumor development in insulin resistance-dependent manner. Oncogene 2016; 35:4459–70 [DOI] [PubMed] [Google Scholar]
- 24.Ande SR, Nguyen KH, Grégoire Nyomba BL, Mishra S. Prohibitin-induced, obesity-associated insulin resistance and accompanying low-grade inflammation causes NASH and HCC. Sci Rep 2016; 6:23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen KH, Ande SR, Mishra S. Obesity-related abnormalities couple environmental triggers with genetic susceptibility in adult-onset T1D. Biochem Biophys Res Commun 2016; 470:94–100 [DOI] [PubMed] [Google Scholar]
- 26.Nijtmans LG, de Jong L, Artal Sanz M, Coates PJ, Berden JA, Back JW, Muijsers AO, van der Spek H, Grivell LA. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J 2000; 19:2444–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra S, Ande SR, Nyomba BL. The role of prohibitin in cell signaling. FEBS J 2010; 277:3937–46 [DOI] [PubMed] [Google Scholar]
- 28.Mishra S, Nyomba BG. Prohibitin – at the crossroads of obesity-linked diabetes and cancer. Exp Biol Med 2017; 242:1170–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Womersley J. A comparison of the skinfold method with extent of overweight and various weight–height relationships in the assessment of obesity. Br J Nutr 1977; 38:271–84 [DOI] [PubMed] [Google Scholar]
- 30.Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, Skinner JS, Bouchard C, Wilmore JH. The effect of sex, age and race on estimating percentage body fat from body mass index: the heritage family study. Int J Obes Relat Obes 2002; 26:789–96 [DOI] [PubMed] [Google Scholar]
- 31.Maynard LM, Wisemandle W, Roche AF, Chumlea WC, Guo SS, Siervogel RM. Childhood body composition in relation to body mass index. Pediatrics 2001; 107:344–50 [DOI] [PubMed] [Google Scholar]
- 32.Hattori K, Tahara Y, Moji K, Aoyagi K, Furusawa T. Chart analysis of body composition change among pre- and postadolescent Japanese subjects assessed by underwater weighing method. Int J Obes Relat Obes 2004; 28:520–4 [DOI] [PubMed] [Google Scholar]
- 33.Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab 2007; 21:415–30 [DOI] [PubMed] [Google Scholar]
- 34.Toth MJ, Tchernof A, Sites CK, Poehlman ET. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Obes 2000; 24:226–31 [DOI] [PubMed] [Google Scholar]
- 35.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes 2008; 32:949–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elbers JM, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol 1999; 276:E317–25 [DOI] [PubMed] [Google Scholar]
- 37.Vague J. La différentiation sexuelle. Facteur determinant des formes de l′obesité. Press Med 1947; 30:339–40 [PubMed] [Google Scholar]
- 38.Vague J. The degree of masculine differentiation of obesity. A factor determining predisposition to diabetes. Am J Clin Nutr 1956; 4:20–34 [DOI] [PubMed] [Google Scholar]
- 39.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes 2010; 34:949–59 [DOI] [PubMed] [Google Scholar]
- 40.Pi-Sunyer FX. The epidemiology of central fat distribution in relation to disease. Nutr Rev 2004; 62:S120–6 [DOI] [PubMed] [Google Scholar]
- 41.Fried SK, Kral JG. Sex differences in regional distribution of fat cell size and lipoprotein lipase activity in morbidly obese patients. Int J Obes 1987; 11:129–40 [PubMed] [Google Scholar]
- 42.Edens NK, Fried SK, Kral JG, Hirsch J, Leibel RL. In vitro lipid synthesis in human adipose tissue from three abdominal sites. Am J Physiol 1993; 265:E374–9 [DOI] [PubMed] [Google Scholar]
- 43.Pedersen O, Hjøllund E, Lindskov HO. Insulin binding and action on fat cells from young healthy females and males. Am J Physiol 1982; 243:E158–67 [DOI] [PubMed] [Google Scholar]
- 44.Foley JE, Kashiwagi A, Chang H, Huecksteadt TP, Lillioja S, Verso MA, Reaven G. Sex difference in insulin-stimulated glucose transport in rat and human adipocytes. Am J Physiol 1984; 246:E211–5 [DOI] [PubMed] [Google Scholar]
- 45.Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 2007; 56:1369–75 [DOI] [PubMed] [Google Scholar]
- 46.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ 2015; 6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 2013; 34:309–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson LA, McTernan PG, Barnett AH, Kumar S. The effects of androgens and estrogens on preadipocyte proliferation in human adipose tissue: influence of gender and site. J Clin Endocrinol Metab 2001; 86:5045–51 [DOI] [PubMed] [Google Scholar]
- 49.Gupta V, Bhasin S, Guo W, Singh R, Miki R, Chauhan P, Choong K, Tchkonia T, Lebrasseur NK, Flanagan JN, Hamilton JA, Viereck JC, Narula NS, Kirkland JL, Jasuja R. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Mol Cell Endocrinol 2008; 296:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blouin K, Nadeau M, Perreault M, Veilleux A, Drolet R, Marceau P, Mailloux J, Luu-The V, Tchernof A. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol 2010; 72:176–88 [DOI] [PubMed] [Google Scholar]
- 51.Rice SP, Zhang L, Grennan-Jones F, Agarwal N, Lewis MD, Rees DA, Ludgate M. Dehydroepiandrosterone (DHEA) treatment in vitro inhibits adipogenesis in human omental but not subcutaneous adipose tissue. Mol Cell Endocrinol 2010; 320:51–7 [DOI] [PubMed] [Google Scholar]
- 52.Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Muller J, Skakkebaek NE, Heiman ML, Birkett M, Attanasio AM, Kiess W, Rascher W. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab 1997; 82:2904–10 [DOI] [PubMed] [Google Scholar]
- 53.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, Matsuda M, Kondo H, Furuyama N, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 2002; 51:2734–41 [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Cuenca S, Pujol E, Justo R, Frontera M, Oliver J, Gianotti M, Roca P. Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. J Biol Chem 2002; 277:42958–63 [DOI] [PubMed] [Google Scholar]
- 55.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360:1509–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. 2012; 15:432–7 [DOI] [PubMed] [Google Scholar]
- 57.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol 2011; 6:275–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodríguez-Prados JC, Través PG, Cuenca J, Rico D, Aragonés J, Martín-Sanz P, Cascante M, Boscá L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol 2010; 185:605–14 [DOI] [PubMed] [Google Scholar]
- 59.Keselman A, Fang X, White PB, Heller NM. Estrogen signaling contributes to sex differences in macrophage polarization during asthma. J Immunol 2017; 199:1573–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Navarro G, Allard C, Xu W, Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity 2015; 23:713–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ande SR, Nguyen KH, Nyomba BLG, Mishra S. Prohibitin in adipose and immune functions. Trends Endocrinol Metab 2016; 27:531–41 [DOI] [PubMed] [Google Scholar]
- 62.Kang T, Lu W, Xu W, Anderson L, Bacanamwo M, Thompson W, Chen YE, Liu D. MicroRNA-27 (miR-27) targets prohibitin and impairs adipocyte differentiation and mitochondrial function in human adipose-derived stem cells. J Biol Chem 2013; 288:34394–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ande SR, Xu YXZ, Mishra S. Prohibitin: a potential therapeutic target in tyrosine kinase signaling. Sig Transduct Target Ther 2017; 2:17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, Scherer PE. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med 2012; 18:1539–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu Y, Luo L, Luo N, Garvey WT. Lipid metabolism mediated by adipocyte lipid binding protein (ALBP/aP2) gene expression in human THP-1 macrophages. Atherosclerosis 2006; 188:102–11 [DOI] [PubMed] [Google Scholar]
- 66.Rolph MS, Young TR, Shum BO, Gorgun CZ, Schmitz-Peiffer C, Ramshaw IA, Hotamisligil GS, Mackay CR. Regulation of dendritic cell function and T cell priming by the fatty acid binding protein AP2. J Immunol 2006; 177:7794–801 [DOI] [PubMed] [Google Scholar]
- 67.Xu YXZ, Ande SR, Mishra S. Gonadectomy in Mito-Ob mice revealed a sex-dimorphic relationship between prohibitin and sex steroids in adipose tissue biology and glucose homeostasis. Biol Sex Differ 2018; 9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong P, Jiang L, Liu J, Wu Z, Guo S, Zhang Z, Zhou F, Liu Z. Induction of paclitaxel resistance by Era mediated prohibitin mitochondrial-nuclear shuttling. PLoS One 2013; 8:e83519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kasashima K, Sumitani M, Satoh M, Endo H. Human prohibitin 1 maintains the organization and stability of the mitochondrial nucleoids. Exp Cell Res 2008; 314:988–96 [DOI] [PubMed] [Google Scholar]
- 70.Theiss AL, Vijay-Kumar M, Obertone TS, Jones DP, Hansen JM, Gewirtz AT, Merlin D, Sitaraman SV. Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology 2009; 137:199–208, 208.e1–e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol 2001; 2:777–80 [DOI] [PubMed] [Google Scholar]
- 72.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 2008; 8:737–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guerra-Silveira F, Abad-Franch F. Sex bias in infectious disease epidemiology: patterns and processes. PLoS One 2013; 8:e62390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Markle JG, Fish EN. SeXX matters in immunity. Trends Immunol 2014; 35:97–104 [DOI] [PubMed] [Google Scholar]
- 75.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis 2010; 10:338–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity 2013; 38:633–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seillet C, Laffont S, Trémollières F, Rouquié N, Ribot C, Arnal JF, Douin-Echinard V, Gourdy P, Guéry JC. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood 2012; 119:454. [DOI] [PubMed] [Google Scholar]
- 78.Calippe B, Douin-Echinard V, Laffargue M, Laurell H, Rana-Poussine V, Pipy B, Guéry JC, Bayard F, Arnal JF, Gourdy P. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. J Immunol 2008; 180:7980–8 [DOI] [PubMed] [Google Scholar]
- 79.Gourdy P, Araujo LM, Zhu R, Garmy-Susini B, Diem S, Laurell H, Leite-de-Moraes M, Dy M, Arnal JF, Bayard F, Herbelin A. Relevance of sexual dimorphism to regulatory T cells: estradiol promotes IFN-gamma production by invariant natural killer T cells. Blood 2005; 105:2415–2 [DOI] [PubMed] [Google Scholar]
- 80.Jares P, Colomer D, Campo E. Molecular pathogenesis of mantle cell lymphoma. J Clin Invest 2012; 122:3416–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gladden AB, Woolery R, Aggarwal P, Wasik MA, Diehl JA. Expression of constitutively nuclear cyclin D1 in murine lymphocytes induces B-cell lymphoma. Oncogene 2006; 25:998–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Römer K, Pfreundschuh M. How do estrogens control lymphoma? Blood 2014; 123:1980–1 [DOI] [PubMed] [Google Scholar]
- 83.Yakimchuk K, Hasni MS, Guan J, Chao MP, Sander B, Okret S. Inhibition of lymphoma vascularization and dissemination by estrogen receptor β agonists. Blood 2014; 123:2054–61 [DOI] [PubMed] [Google Scholar]
- 84.Gamble SC, Chotai D, Odontiadis M, Dart DA, Brooke GN, Powell SM, Reebye V, Varela-Carver A, Kawano Y, Waxman J, Bevan CL. Prohibitin, a protein downregulated by androgens, represses androgen receptor activity. Oncogene 2007; 26:1757–68 [DOI] [PubMed] [Google Scholar]
- 85.Hammond GL. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol 2016; 230:R13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Molina L, Figueroa CD, Bhoola KD, Ehrenfeld P. GPER-1/GPR30 a novel estrogen receptor sited in the cell membrane: therapeutic coupling to breast cancer. Expert Opin Ther Targets 2017; 21:755–66 [DOI] [PubMed] [Google Scholar]
- 87.Vail G, Roepke TA. Membrane-initiated estrogen signaling via Gq-coupled GPCR in the central nervous system. Steroids 2018; 10.1016/j.steroids.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Torres MJ, Ryan TE, Lin CT, Zeczycki TN, Neufer PD. Impact of 17β-estradiol on complex I kinetics and H(2)O(2) production in liver and skeletal muscle mitochondria. J Biol Chem 2018; 293:16889–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oo PS, Yamaguchi Y, Sawaguchi A, Tin Htwe Kyaw M, Choijookhuu N, Noor Ali M, Srisowanna N, Hino SI, Hishikawa Y. Estrogen regulates mitochondrial morphology through phosphorylation of dynamin-related protein 1 in MCF7 human breast cancer cells. Acta Histochem Cytochem 2018; 51:21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zi Xu YX, Ande SR, Mishra S. Prohibitin: a new player in immunometabolism and in linking obesity and inflammation with cancer. Cancer Lett 2018; 415:208–16 [DOI] [PubMed] [Google Scholar]
- 91.Jitrapakdee S, Vidal-Puig A, Wallace JC. Anaplerotic roles of pyruvate carboxylase in mammalian tissues. Cell Mol Life Sci 2006; 63:843–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amengual-Cladera E, Lladó I, Gianotti M, Proenza AM. Retroperitoneal white adipose tissue mitochondrial function and adiponectin expression in response to ovariectomy and 17b-estradiol replacement. Steroids 2012; 77:659–65 [DOI] [PubMed] [Google Scholar]
- 93.Zamora-Mendoza R, Rosas-Vargas H, Ramos-Cervantes MT, Garcia-Zuniga P, Perez-Lorenzana H, Mendoza-Lorenzo P, Perez-Ortiz AC, Estrada-Mena FJ, Miliar-Garcia A, Lara-Padilla E, Ceballos G, Rodriguez A, Villarreal F, Ramirez-Sanchez I. Dysregulation of mitochondrial function and biogenesis modulators in adipose tissue of obese children. Int J Obes Relat Metab Disord 2018; 42:618–24 [DOI] [PubMed] [Google Scholar]
- 94.Hino K, Nishina S, Sasaki K, Hara Y. Mitochondrial damage and iron metabolic dysregulation in hepatitis C virus infection. Free Radic Biol Med 2018; 10.1016/j.freeradbiomed.2018.09.044. [DOI] [PubMed] [Google Scholar]
- 95.King AL, Mantena SK, Andringa KK, Millender-Swain T, Dunham-Snary KJ, Oliva CR, Griguer CE, Bailey SM. The methyl donor S-adenosylmethionine prevents liver hypoxia and dysregulation of mitochondrial bioenergetic function in a rat model of alcohol-induced fatty liver disease. Redox Biol 2016; 9:188–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Justo R, Frontera M, Pujol E, Rodriguez-Cuenca S, Llado I, Garcia-Palmer FJ, Roca P, Gianotti M. Gender-related differences in morphology and thermogenic capacity of brown adipose tissue mitochondrial subpopulations. Life Sci 2005; 76:1147–58 [DOI] [PubMed] [Google Scholar]
- 97.Nadal-Casellas A, Amengual-Cladera E, Proenza AM, Llado I, Gianotti M. Longterm high-fat-diet feeding impairs mitochondrial biogenesis in liver of male and female rats. Cell Physiol Biochem 2010; 26:291–302 [DOI] [PubMed] [Google Scholar]
- 98.Colom B, Oliver J, Roca P, Garcia-Palmer FJ. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res 2007; 74:456–65 [DOI] [PubMed] [Google Scholar]
- 99.Guevara R, Santandreu FM, Valle A, Gianotti M, Oliver J, Roca P. Sex-dependent differences in aged rat brain mitochondrial function and oxidative stress. Free Radic Biol Med 2009; 46:169–75 [DOI] [PubMed] [Google Scholar]
- 100.Gomez-Perez Y, Amengual-Cladera E, Catala-Niell A, Thomas-Moya E, Gianotti M, Proenza AM, Lladó I. Gender dimorphism in high-fat-diet-induced insulin resistance in skeletal muscle of aged rats. Cell Physiol Biochem 2008; 22:539–48 [DOI] [PubMed] [Google Scholar]
- 101.Quevedo S, Roca P, Pico C, Palou A. Sex-associated differences in cold-induced UCP1 synthesis in rodent brown adipose tissue. Pflugers Arch 1998; 436:689–95 [DOI] [PubMed] [Google Scholar]
- 102.Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, Klinge CM. Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol Endocrinol 2008; 22:609–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dieudonne MN, Leneveu MC, Giudicelli Y, Pecquery R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol 2004; 286:C655–61 [DOI] [PubMed] [Google Scholar]
- 104.Smiraglia D, Kulawiec M, Bistulfi GL, Ghoshal S, Singh KK. A novel role for mitochondria in regulating epigenetic modification in the nucleus. Cancer Biol Ther 2008; 7:1182–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Balaton BP, Cotton AM, Brown CJ. Derivation of consensus inactivation status for X-linked genes from genome-wide studies. Biol Sex Differ 2015; 6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucl Acids Res 2016; 44:D1251–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 2006; 312:1669–72 [DOI] [PubMed] [Google Scholar]
- 108.Jia M, Dahlman-Wright K, Gustafsson JÅ. Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab 2015; 29:557–68 [DOI] [PubMed] [Google Scholar]
- 109.Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol 2018; 9:2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Viña J, Gambini J, García-García FJ, Rodriguez-Mañas L, Borrás C. Role of oestrogens on oxidative stress and inflammation in ageing. Horm Mol Biol Clin Investig 2013; 16:65–72 [DOI] [PubMed] [Google Scholar]
- 111.Zhu L, Martinez MN, Emfinger CH, Palmisano BT, Stafford JM. Estrogen signaling prevents diet-induced hepatic insulin resistance in male mice with obesity. Am J Physiol Endocrinol Metab 2014; 306:E188–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006; 83:461S–5S [DOI] [PubMed] [Google Scholar]
- 113.Nishimura S, Manab I, Nagai R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov Med 2009; 8:55–60 [Database] [PubMed] [Google Scholar]
- 114.O'Sullivan J, Lysaght J, Donohoe CL, Reynolds JV. Obesity and gastrointestinal cancer: the interrelationship of adipose and tumour microenvironments. Nat Rev Gastroenterol Hepatol 2018; 15:699–714 [DOI] [PubMed] [Google Scholar]
- 115.Santos RS, de Fatima LA, Frank AP, Carneiro EM, Clegg DJ. The effects of 17 alpha-estradiol to inhibit inflammation in vitro. Biol Sex Differ 2017; 8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev 2002; 23:90–119 [DOI] [PubMed] [Google Scholar]
- 117.Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, Navas CR4, Gordillo R, Neinast M, Kalainayakan SP, Li DL, Gao Y, Yi CX, Hahner L, Palmer BF, Tschöp MH, Clegg DJ. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Rep 2014; 9:633–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Drew BG, Hamidi H, Zhou Z, Villanueva CJ, Krum SA, Calkin AC, Parks BW, Ribas V, Kalajian NY, Phun J, Daraei P, Christofk HR, Hewitt SC, Korach KS, Tontonoz P, Lusis AJ, Slamon DJ, Hurvitz SA, Hevener AL. Estrogen receptor (ER)α-regulated lipocalin 2 expression in adipose tissue links obesity with breast cancer progression. J Biol Chem 2015; 290:5566–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Monteiro R, Teixeira D, Calhau C. Estrogen signaling in metabolic inflammation. Mediat Inflamm 2014; 2014:615917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hughes GC, Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat Rev Rheumatol 2014; 10:740–51 [DOI] [PubMed] [Google Scholar]
- 121.Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest 1999; 22:110–6 [PubMed] [Google Scholar]
- 122.Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes 2010; 17:224–32 [DOI] [PubMed] [Google Scholar]
- 123.Yu IC, Lin H-Y, Sparks JD, Yeh S, Chang C. Androgen receptor roles in insulin resistance and obesity in males: Th linkage of androgen-deprivation therapy and metabolic syndrome. Diabetes 2014; 63:3180–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer 2006; 106:581–8 [DOI] [PubMed] [Google Scholar]
- 125.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol 2009; 181:1998–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.La VS, Calogero AE, D’Agata R, D, Mauro M, Tumino S, Condorelli R, Lanzafame F, Finocchiaro C, Giammusso B, Vicari E. Testosterone therapy improves the clinical response to conventional treatment for male patients with metabolic syndrome associated to late onset hypogonadism. Minerva Endocrinol 2008; 33:159–67 [PubMed] [Google Scholar]
- 127.Saad F, Gooren LJ, Haider A, Yassin A. A dose-response study of testosterone on sexual dysfunction and features of the metabolic syndrome using testosterone gel and parenteral testosterone undecanoate. J Androl 2008; 29:102–5 [DOI] [PubMed] [Google Scholar]
- 128.Sekeris CE. The mitochondrial genome: a possible primary site of action of steroid hormones. In Vivo 1990; 4:317–20 [PubMed] [Google Scholar]
- 129.Demonacos C, Djordjevic-Markovic R, Tsawdaroglou N, Sekeris CE. The mitochondrion as a primary site of action of glucocorticoids: the interaction of the glucocorticoid receptor with mitochondrial DNA sequences showing partial similarity to the nuclear glucocorticoid responsive elements. J Steroid Biochem Mol Biol 1995; 55:43–55 [DOI] [PubMed] [Google Scholar]
- 130.Han J, Yu C, Souza RF, Theiss AL. Prohibitin 1 modulates mitochondrial function of Stat3. Cell Signal 2014; 26:2086–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci U S A 1994; 91:1309–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kang D, Kim SH, Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion 2007; 7:39–44 [DOI] [PubMed] [Google Scholar]
- 133.Naviaux RK. Mitochondrial control of epigenetics. Cancer Biol Ther 2008; 7:1191–3 [DOI] [PubMed] [Google Scholar]
- 134.Song F, Smith JF, Kimura MT, Morrow AD, Matsuyama T, Nagase H, Held WA. Association of tissue-specific differentially methylated regions (TDMs) with differential gene expression. Proc Natl Acad Sci U S A 2005; 102:3336–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chabi B, Mousson de Camaret B, Duborjal H, Issartel JP, Stepien G. Quantification of mitochondrial DNA deletion, depletion and overreplication: application to diagnosis. Clin Chem 2003; 49:1309–17 [DOI] [PubMed] [Google Scholar]
- 136.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005; 434:400–4 [DOI] [PubMed] [Google Scholar]
- 137.Bianchi I, Lleo A, Gershwin ME, Invernizzi P. The X chromosome and immune associated genes. J Autoimmun 2012; 38:J187–92 [DOI] [PubMed] [Google Scholar]
- 138.Deng X, Berletch JB, Nguyen DK, Disteche CM. X chromosome regulation: diverse patterns in development, tissues and disease. Nat Rev Genet 2014; 15:367–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa A, Searle S, Barnes I, Bignell A, Boychenko V, Hunt T, Kay M, Mukherjee G, Rajan J, Despacio-Reyes G, Saunders G, Steward C, Harte R, Lin M, Howald C, Tanzer A, Derrien T, Chrast J, Walters N, Balasubramanian S, Pei B, Tress M, Rodriguez JM, Ezkurdia I, van Baren J, Brent M, Haussler D, Kellis M, Valencia A, Reymond A, Gerstein M, Guigó R, Hubbard TJ. GENCODE: the reference human genome annotation for the ENCODE project. Genome Res 2012; 22:1760–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Harrow J, Denoeud F, Frankish A, Reymond A, Chen CK, Chrast J, Lagarde J, Gilbert JG, Storey R, Swarbreck D, Rossier C, Ucla C, Hubbard T, Antonarakis SE, Guigo R. GENCODE: producing a reference annotation for ENCODE. Genome Biol 2006; 7:S4.1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guo X, Su B, Zhou Z, Sha J. Rapid evolution of mammalian X-linked testis microRNAs. BMC Genom 2009; 10:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu Q, Wu A, Tesmer L, Ray D, Yousif N, Richardson B. Demethylation of CD40LG on the inactive X in T cells from women with lupus. J Immunol 2007; 179:6352–8 [DOI] [PubMed] [Google Scholar]
- 143.Zhao M, Sun Y, Gao F, Wu X, Tang J, Yin H, Luo Y, Richardson B, Lu Q. Epigenetics and SLE: RFX1 downregulation causes CD11a and CD70 overexpression by altering epigenetic modifications in lupus CD4þ T cells. J Autoimmun 2010; 35:58–69 [DOI] [PubMed] [Google Scholar]
- 144.Patel DR, Richardson BC. Epigenetic mechanisms in lupus. Curr Opin Rheumatol 2010; 22:478–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim DK, Kim HS, Kim AR, Jang GH, Kim HW, Park YH, Kim B, Park YM, Beaven MA, Kim YM, Choi WS. The scaffold protein prohibitin is required for antigen-stimulated signaling in mast cells. Sci Signal 2013; 6:ra80. [DOI] [PubMed] [Google Scholar]
- 146.Lucas CR, Cordero-Nieves HM, Erbe RS, McAlees JW, Bhatia S, Hodes RJ, Campbell KS, Sanders VM. Prohibitins and the cytoplasmic domain of CD86 cooperate to mediate CD86 signaling in B lymphocytes. J Immunol 2013; 190:723–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ross JA, Nagy ZS, Kirken RA. The PHB1/2 phosphocomplex is required for mitochondrial homeostasis and survival of human T cells. J Biol Chem 2008; 283:4699–713 [DOI] [PubMed] [Google Scholar]
- 148.Merkwirth C, Martinelli P, Korwitz A, Morbin M, Brönneke HS, Jordan SD, Rugarli EI, Langer T. Loss of prohibitin membrane scaffolds impairs mitochondrial architecture and leads to tau hyperphosphorylation and neurodegeneration. PLoS Genet 2012; 8:e1003021. [DOI] [PMC free article] [PubMed] [Google Scholar]


