Abstract
Background
Admission blood glucose (BG) level is a predictor of mortality in critically ill patients with various conditions. However, limited data are available regarding this relationship in critically ill patients with cardiovascular diseases according to diabetic status.
Methods
A total of 1,780 patients (595 with diabetes) who were admitted to cardiac intensive care unit (CICU) were enrolled from a single center registry. Admission BG level was defined as maximal serum glucose level within 24 hours of admission. Patients were divided by admission BG level: group 1 (< 7.8 mmol/L), group 2 (7.8–10.9 mmol/L), group 3 (11.0–16.5 mmol/L), and group 4 (≥ 16.6 mmol/L).
Results
A total of 105 patients died in CICU (62 non-diabetic patients [5.2%] and 43 diabetic patients [7.9%]; P = 0.105). The CICU mortality rate increased with admission BG level (1.7%, 4.8%, 10.3%, and 18.8% from group 1 to group 4, respectively; P < 0.001). On multivariable analysis, hypertension, mechanical ventilator, continuous renal replacement therapy, acute physiology and chronic health evaluation II (APACHE II) score, and admission BG level significantly influenced CICU mortality in non-diabetic patients (group 1 vs. group 3: hazard ratio [HR], 3.31; 95% confidence interval [CI], 1.47–7.44; P = 0.004; group 1 vs. group 4: HR, 6.56; 95% CI, 2.76–15.58; P < 0.001). However, in diabetic patients, continuous renal replacement therapy and APACHE II score influenced CICU mortality but not admission BG level.
Conclusion
Admission BG level was associated with increased CICU mortality in critically ill, non-diabetic patients admitted to CICU but not in diabetic patients.
Keywords: Blood Glucose, Cardiac Intensive Care Unit, Diabetes, Prognosis
Graphical Abstract
INTRODUCTION
Hyperglycemia is common in critically ill patients irrespective of the presence of diabetes mellitus,1 which is called stress hyperglycemia. Stress hyperglycemia has been regarded as a poor prognostic factor influencing morbidity and mortality of critically ill patients. The mechanism has been explained by stress hormone secretion, which reflects severity of the disease,2,3 and by the hyperglycemia itself, which exacerbates the cytokine, inflammatory, and oxidative stress response, potentially setting up a vicious cycle.4,5 Previous studies conducted in various critically ill patients showed that admission blood glucose (BG) level was related to prognosis6; however, most previous studies regarding cardiovascular disease focused on acute coronary syndrome7 or decompensated heart failure.8 Furthermore, the prognostic role of BG level may differ in diabetic versus non-diabetic patients because BG level inevitably depends on the degree of glucose control with glucose-lowering strategies in diabetic patients. Several studies have reported that hyperglycemia is associated with clinical outcomes irrespective of the presence of diabetes mellitus,9 while some studies have reported that the relationship was valid only in non-diabetic patients.7 Meanwhile, there are concerns that mild to moderate stress hyperglycemia might be protective since it could provide a source of fuel for the immune system and brain and contribute to the survival of critically ill patients.10 Therefore, we sought to investigate the association between admission BG level and clinical outcomes in critically ill patients with various cardiovascular diseases who were admitted to cardiac intensive care unit (CICU) according to the presence or absence of diabetic mellitus.
METHODS
Study population
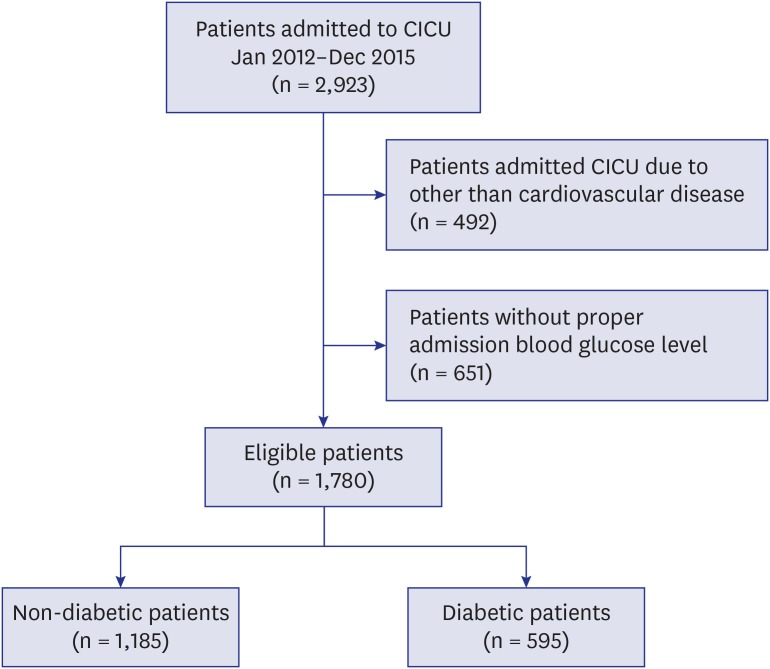
We consecutively screened patients who were admitted to the CICU in Samsung Medical Center from January 2013 to December 2015. There were 2,923 patients admitted to the CICU of our hospital during the study period. We excluded 492 patients admitted to the CICU due to diseases other than cardiovascular problems and 651 patients who did not have admission BG level data. Finally, 1,780 patients were included in this study (Fig. 1). Then, we classified study populations by clinically important glucose levels according to the guidelines from American Diabetes Association11; group 1 (< 7.8 mmol/L), group 2 (7.8 mmol/L to 10.9 mmol/L), group 3 (11.0 mmol/L to 16.5 mmol/L), and group 4 (≥ 16.6 mmol/L).
Fig. 1. Flow chart of patient recruitment from the single center registry of CICU.
CICU = cardiac intensive care unit, BG = blood glucose.
Definitions and outcomes
Underlying diseases including hypertension, cerebrovascular disease, chronic kidney disease, liver cirrhosis, and malignancy were extracted from the electronic medical records. Diabetes mellitus was diagnosed at a fasting BG level ≥ 7.0 mmol/L or a random glucose level ≥ 11.1 mmol/L or glycated hemoglobin ≥ 6.5%.12 Patients who were taking insulin or oral hypoglycemic agents were also defined as having diabetes mellitus. For evaluation of disease severity, we calculated acute physiology and chronic health evaluation II (APACHE II) scores of study participants. Admission BG level was defined as the maximum serum glucose level within 24 hours of CICU admission.
The primary outcome was CICU mortality. Use of intra-aortic balloon pump, extracorporeal membrane oxygenation, mechanical ventilator, renal replacement therapy, or heart transplantation was also assessed.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as frequency and percentage. Continuous variables between non-diabetic and diabetic patients were compared using the t-test or Mann-Whitney U test. Categorical variables were compared using χ2 or Fisher's exact tests. Event-free survival was estimated by the Kaplan-Meier method and was compared using the log-rank test. Cox proportional hazard regression analysis was performed to evaluate the influence of admission BG level on CICU mortality in non-diabetic patients and diabetic patients. Parameters that were revealed to influence patient outcomes in the Cox regression model were adjusted in the final model. Disease severity was assessed by APACHE II score, and patients were divided into two groups using the cutoff point of 16.5, which was defined by the Yuden index for multivariable cox regression analysis. We used SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.4.0 (R Foundation, Vienna, Austria) for Windows for all statistical analyses, with P < 0.05 considered statistically significant.
Ethics statement
The Institutional Review Board (IRB) of Samsung Medical Center approved the study protocol (IRB No. 2016-05-125) and waived the requirement for informed consent.
RESULTS
Baseline characteristics of the patients
Among the 1,780 patients in this study, 595 (33.4%) had diabetes mellitus; the mean age of patients was 65 ± 14 years. Baseline characteristics are shown in Table 1. The most common cause of admission was acute coronary syndrome (935, 52.5%), and other leading causes were heart failure (434, 24.4%) and cardiac arrhythmia (211, 11.9%). When the cause of admission was compared between non-diabetic and diabetic patients, acute coronary syndrome and heart failure were more common in diabetic patients. APACHE II score and the frequency of shock development during CICU admission were also higher in diabetic patients. The admission BG level for all patients was 10.0 ± 4.8 mmol/L; it was 8.3 ± 2.3 mmol/L in non-diabetic patients and 13.2 ± 5.6 mmol/L in diabetic patients (P < 0.001).
Table 1. Baseline characteristics of non-diabetic and diabetic patients.
| Characteristics | Non-diabetes (1,185) | Diabetes (595) | P value | |
|---|---|---|---|---|
| Gender, men | 782 (66.0) | 393 (66.1) | 1.000 | |
| Age, yr | 62.62 ± 15.43 | 69.01 ± 11.12 | < 0.001 | |
| BMI, kg/m2 | 23.77 ± 3.85 | 23.92 ± 3.69 | 0.425 | |
| Hypertension | 498 (42.0) | 442 (74.3) | < 0.001 | |
| Cerebrovascular accident | 60 (5.1) | 54 (9.1) | 0.002 | |
| Chronic obstructive pulmonary disease | 49 (4.1) | 33 (5.5) | 0.222 | |
| Chronic kidney disease | 106 (8.9) | 113 (19.0) | < 0.001 | |
| Liver cirrhosis | 22 (1.9) | 24 (4.0) | 0.010 | |
| Cancer | 82 (6.9) | 44 (7.4) | 0.787 | |
| Admission BG, mmol/L | 8.3 ± 2.3 | 13.2 ± 5.6 | < 0.001 | |
| Cause of admission | < 0.001 | |||
| Acute coronary syndrome | 607 (51.2) | 328 (55.1) | ||
| Heart failure | 274 (23.1) | 160 (26.9) | ||
| Arrhythmia | 137 (11.6) | 74 (12.4) | ||
| Others | 167 (14.1) | 33 (5.5) | ||
| APACHE II score | 10.75 ± 7.44 | 14.40 ± 7.92 | < 0.001 | |
| Shock | 232 (19.6) | 159 (26.7) | 0.001 | |
Data are presented as mean ± standard deviation or number (%).
BMI = body mass index, BG = blood glucose, APACHE II = acute physiology and chronic health evaluation II score.
For purposes of the study, patients were divided into four groups according to admission BG level. The prevalence of shock increased according to admission BG level (11.8%, 22.3%, 40.8%, and 61.9%, P < 0.001 in non-diabetic patients; 18.1%, 19.6%, 25.5%, and 45.5%, P < 0.001 in diabetic patients). APACHE II scores were also higher in the patient group with higher admission BG levels, both in non-diabetic and diabetic patients (P < 0.001, in both). The difference of baseline characteristics by admission BG level is in Supplementary Table 1.
Clinical outcomes according to admission BG level
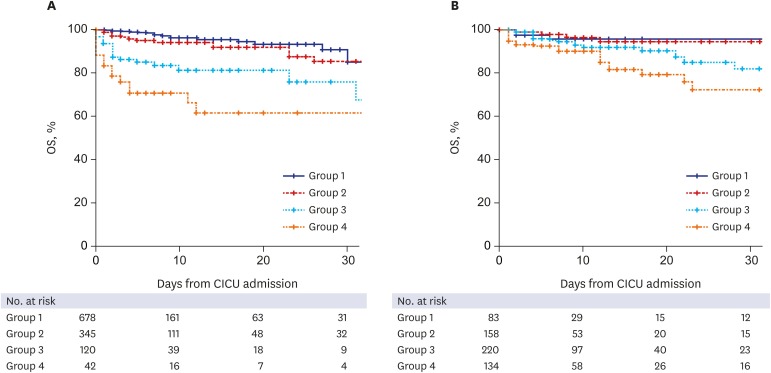
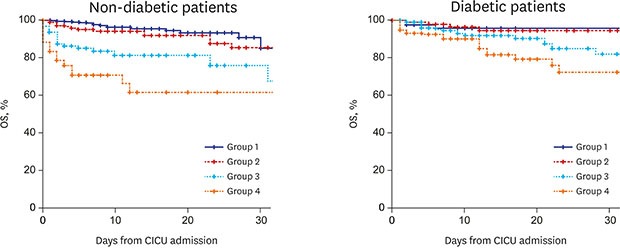
During the study period, median duration of hospital stay was 6 (3–12) days in all patients; it was 5 (3–10) days in non-diabetic patients and 7 (4–15) days in diabetic patients (P < 0.001). Median duration of CICU stay was 2 (1–3) days; 2 (1–3) days for non-diabetic patients and 2 (1–4) days for diabetic patients (P < 0.001). CICU mortality occurred in 105 patients (5.9%), which included 62 (5.2%) non-diabetic patients and 43 (7.2%) diabetic patients (P = 0.105). In non-diabetic patients, the CICU mortality rate was higher in patient groups with higher admission BG levels (1.5%, 5.5%, 15.8%, and 33.3% in groups 1 to 4; P < 0.001). In diabetic patients, although the cumulative CICU mortality gradually increased, it was lacking in statistical significance (3.6%, 3.2%, 7.3%, and 14.2% in groups 1 to 4; P = 0.279) (Fig. 2). The prevalence of patients who received extracorporeal membrane oxygenation, mechanical ventilation, continuous renal replacement therapy, and cardiac transplantation increased with the increase in admission BG level in the non-diabetic patients. However, there was no significant increase in BG level with the use of extracorporeal membrane oxygenation and cardiac transplantation in diabetic patients, unlike mechanical ventilation and continuous renal replacement therapy (Table 2).
Fig. 2. Kaplan-Meier curves for mortality during the follow-up. (A) non-diabetic patients, and (B) diabetic patients. Patients were divided into four groups by admission BG level both in non-diabetic and diabetic patients respectively; group 1 (< 7.8 mmol/L), group 2 (7.8 mmol/L to 10.9 mmol/L), group 3 (11.0 mmol/L to 16.5 mmol/L), and group 4 (≥ 16.6 mmol/L).
OS = overall survival, CICU = cardiac intensive care unit, BG = blood glucose.
Table 2. Frequencies of organ support by the different baseline glucose levels in non-diabetic and diabetic patients.
| Variables | Group 1 | Group 2 | Group 3 | Group 4 | P value | |
|---|---|---|---|---|---|---|
| Non-diabetes | ||||||
| Extracorporeal membrane oxygenation | 15 (2.2) | 16 (4.6) | 14 (11.7) | 9 (21.4) | < 0.001 | |
| Mechanical ventilator | 57 (8.4) | 53 (15.4) | 48 (40.0) | 24 (57.1) | < 0.001 | |
| Continuous renal replacement therapy | 28 (4.1) | 24 (7.0) | 22 (18.3) | 11 (26.2) | < 0.001 | |
| Heart transplantation | 8 (1.2) | 5 (1.4) | 6 (5.0) | 2 (4.8) | 0.014 | |
| Diabetes | ||||||
| Extracorporeal membrane oxygenation | 4 (4.8) | 6 (3.8) | 16 (7.3) | 14 (10.4) | 0.137 | |
| Mechanical ventilator | 7 (8.4) | 28 (17.7) | 52 (23.6) | 52 (38.8) | < 0.001 | |
| Continuous renal replacement therapy | 8 (9.6) | 16 (10.1) | 25 (11.4) | 27 (20.1) | 0.034 | |
| Heart transplantation | 2 (2.4) | 3 (1.9) | 3 (1.4) | 1 (0.7) | 0.777 | |
Data are presented as number (%).
Prognostic significance of admission BG level in non-diabetic and diabetic patients
We performed Cox proportional regression analysis to identify the prognostic significance of admission BG level on CICU mortality in non-diabetic and diabetic patients. Among non-diabetic patients, admission BG level, hypertension, mechanical ventilator support, continuous renal replacement therapy, and APACHE II score were significantly associated with CICU mortality. With group 1 as a reference, adjusted hazard ratios (HRs) of groups 3 and 4 were 3.31 (95% confidence interval [CI], 1.47–7.44; P = 0.004) and 6.56 (95% CI, 2.76–15.58; P < 0.001), respectively. In diabetic patients, continuous renal replacement therapy and APACHE II score were significantly associated with CICU mortality. Although HR for CICU mortality gradually increased in patient groups with an increased admission BG level, the statistical significance was not valid even when mortality was compared between group 1 and group 4 (HR, 1.37; 95% CI, 0.40–4.73; P = 0.616) (Table 3).
Table 3. Multivariable Cox regression analysis of CICU mortality in non-diabetic and diabetic patients.
| Variables | Non-diabetes | Diabetes | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Admission BG level (group 1 as a reference) | |||||||
| Group 2 | 2.13 | 0.97–4.65 | 0.059 | 0.60 | 0.14–2.57 | 0.489 | |
| Group 3 | 3.31 | 1.47–7.44 | 0.004 | 1.05 | 0.30–3.66 | 0.934 | |
| Group 4 | 6.56 | 2.76–15.58 | < 0.001 | 1.37 | 0.40–4.73 | 0.616 | |
| Hypertension | 0.51 | 0.29–0.91 | 0.023 | ||||
| Mechanical ventilator | 2.33 | 1.10–4.95 | 0.028 | ||||
| Continuous renal replacement therapy | 1.87 | 1.02–3.43 | 0.044 | 4.52 | 2.20–9.29 | < 0.001 | |
| APACHE II | 2.66 | 1.26–5.62 | 0.011 | 3.15 | 1.03–9.65 | 0.044 | |
CICU = cardiac intensive care unit, HR = hazard ratio, CI = confidence interval, BG = blood glucose, APACHE II = acute physiology and chronic health evaluation II score.
DISCUSSION
In this study, we evaluated the relationship between admission BG level and CICU mortality in non-diabetic and diabetic patients. There was significant association between baseline BG level and CICU mortality in non-diabetic patients, whereas such an association was not valid in diabetic patients. Admission BG level, APACHE II score, use of mechanical ventilator, renal replacement therapy, and hypertension were prognostic factors for CICU mortality in non-diabetic patients, while only APCHE II score and continuous renal replacement therapy were prognostic factors in diabetic patients.
Stress hyperglycemia is thought to be caused by excessive hepatic glucose production and insulin resistance due to the complex interplay between counter regulatory hormones including cortisol, glucagon, growth hormone, and cytokines.3,13 Since a severe disease status may cause increased stress hormone secretion, hyperglycemia in critically ill patients may be related to more severe disease, which may explain the relation between higher BG level and poor prognosis. In this study, the strong relationship between admission BG level and CICU mortality in non-diabetic patients did not change even when we adjusted for severity of the disease using APACHE II score. The result implies that the relationship between admission BG level and the prognosis of non-diabetic patients is independent from the severity of the disease. This is supported by the finding that hyperglycemia itself in stressful conditions deteriorates endothelial function and promotes pro-inflammatory cytokines.14
The relationship between stress hyperglycemia and prognosis has been documented in various critically ill conditions such as sepsis, postsurgical state, stroke, and trauma.1,15 Similarly, numerous studies were performed in patients with cardiovascular disease,8,16 and they showed a strong relationship between BG level and prognosis. However, these studies regarding the association of admission BG level with clinical outcomes focused primarily on patients with acute coronary syndrome17,18,19 and decompensated heart failure.20,21 Thus, the results of previous studies were not applicable in the overall critically ill patients with cardiovascular disease. Therefore, in this study, we evaluated the association between admission BG level and clinical outcomes in critically ill patients with cardiovascular diseases including pulmonary thromboembolism, acute aortic syndrome, and cardiac arrhythmia as well as acute coronary syndrome and heart failure in diabetic and non-diabetic patients. Then, we found a significant relationship between admission BG level and CICU mortality in this group of patients, especially those without diabetes mellitus.
In this study, the relationship between admission BG level and CICU mortality was different in non-diabetic and diabetic patients. The relationship in diabetic patients was not significant. Some previous studies have reported that the relationship was valid in all patients irrespective of diabetes mellitus,6,9,22 while other studies reported that the relationship was valid only in non-diabetic patients.7,17 Our results suggest that the influence of BG level on prognosis among diabetic patients might be weaker than that in non-diabetic patients. Although the reasons for the relatively weak relationship between BG level and clinical outcomes in diabetic patients is unclear, there are several possible explanations. First, in diabetic patients, BG level is influenced by the degree of BG control before CICU admission, and basal BG level before the cardiac event may also be higher in diabetic patients than in non-diabetic patients, which influences the prognostic role of hyperglycemia during index hospitalization due to critical illness. Second, under critically ill conditions, glucose transporters are abnormally upregulated in non-diabetic patients; therefore, patients become highly susceptible to glucose toxicity.4 However, in diabetic patients, chronic hyperglycemia downregulates the glucose transporters in cells, and the cellular conditioning has been reported to protect cells from acute hyperglycemia-mediated damage4,23 and to contribute to the increase in threshold admission BG level which influences poor prognosis in relation to hyperglycemia. Likewise, since glucose metabolism is very complex in critically ill conditions in diabetic patients and the prognostic role of BG level is still controversial, further large-scale, well-designed studies should be performed to confirm these findings.
This study has several limitations. First, it is possible that patients who present with hyperglycemia may include those with undiagnosed diabetes mellitus. However, diagnostic evaluation for diabetes mellitus is generally performed if patients repetitively present an abnormal BG level during admission. Therefore, the proportion of undiagnosed diabetic patients included in the non-diabetic group is expected to be small. Second, we used a random BG level. Influence of diet or a stressful event was not considered when evaluating admission BG level. Third, patients who presented with hypoglycemia were included in group 1. Hypoglycemia has a negative influence on patient prognosis, which may have resulted in the finding of no significant difference in clinical outcomes between groups 1 and 2 within non-diabetic patients. Finally, we evaluated the relationship between admission BG level and mortality in the acute phase of critical illness, but not the effect of glucose control on patient mortality. From this study, we could not conclude whether glucose control may improve the prognosis for these patients.
In conclusion, admission BG level was associated with increased CICU mortality in critically ill, non-diabetic patients; however, the relationship lacked statistical significance even though CICU mortality numerically increased with baseline BG level in diabetic patients. Further large-scale studies are warranted to confirm these findings, particularly in diabetic patients.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Yang JH.
- Investigation: Park TK, Lee JM, Song YB, Choi JO, Hahn JY, Choi JH, Choi SH, Gwon HC.
- Methodology: Na SJ.
- Validation: Chung CR, Jeon K, Suh GY.
- Writing - original draft: Kim S.
- Writing - review & editing: Yang JH.
SUPPLEMENTARY MATERIAL
Baseline characteristics of the patient by the different admission BG levels
References
- 1.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chernow B, Rainey TG, Lake CR. Endogenous and exogenous catecholamines in critical care medicine. Crit Care Med. 1982;10(6):409–416. doi: 10.1097/00003246-198206000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Barth E, Albuszies G, Baumgart K, Matejovic M, Wachter U, Vogt J, et al. Glucose metabolism and catecholamines. Crit Care Med. 2007;35(9) Suppl:S508–S518. doi: 10.1097/01.CCM.0000278047.06965.20. [DOI] [PubMed] [Google Scholar]
- 4.Vanhorebeek I, Van den Berghe G. Diabetes of injury: novel insights. Endocrinol Metab Clin North Am. 2006;35(4):859–872. x. doi: 10.1016/j.ecl.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53(8):2079–2086. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 6.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 7.Yang JH, Song PS, Song YB, Hahn JY, Choi SH, Choi JH, et al. Prognostic value of admission blood glucose level in patients with and without diabetes mellitus who sustain ST segment elevation myocardial infarction complicated by cardiogenic shock. Crit Care. 2013;17(5):R218. doi: 10.1186/cc13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirakabe A, Hata N, Kobayashi N, Okazaki H, Matsushita M, Shibata Y, et al. Decreased blood glucose at admission has a prognostic impact in patients with severely decompensated acute heart failure complicated with diabetes mellitus. Heart Vessels. 2018;33(9):1008–1021. doi: 10.1007/s00380-018-1151-3. [DOI] [PubMed] [Google Scholar]
- 9.Kataja A, Tarvasmäki T, Lassus J, Cardoso J, Mebazaa A, Køber L, et al. The association of admission blood glucose level with the clinical picture and prognosis in cardiogenic shock - results from the CardShock Study. Int J Cardiol. 2017;226:48–52. doi: 10.1016/j.ijcard.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care. 2013;17(2):305. doi: 10.1186/cc12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2018 . Diabetes Care. 2018;41(Suppl 1):S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 13.Lesur O, Roussy JF, Chagnon F, Gallo-Payet N, Dumaine R, Sarret P, et al. Proven infection-related sepsis induces a differential stress response early after ICU admission. Crit Care. 2010;14(4):R131. doi: 10.1186/cc9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, et al. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest. 2005;115(8):2277–2286. doi: 10.1172/JCI25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rau CS, Wu SC, Chen YC, Chien PC, Hsieh HY, Kuo PJ, et al. Stress-induced hyperglycemia, but not diabetic hyperglycemia, is associated with higher mortality in patients with isolated moderate and severe traumatic brain injury: analysis of a propensity score-matched population. Int J Environ Res Public Health. 2017;14(11):E1340. doi: 10.3390/ijerph14111340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mebazaa A, Gayat E, Lassus J, Meas T, Mueller C, Maggioni A, et al. Association between elevated blood glucose and outcome in acute heart failure: results from an international observational cohort. J Am Coll Cardiol. 2013;61(8):820–829. doi: 10.1016/j.jacc.2012.11.054. [DOI] [PubMed] [Google Scholar]
- 17.Kolman L, Hu YC, Montgomery DG, Gordon K, Eagle KA, Jackson EA. Prognostic value of admission fasting glucose levels in patients with acute coronary syndrome. Am J Cardiol. 2009;104(4):470–474. doi: 10.1016/j.amjcard.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Vis MM, Sjauw KD, van der Schaaf RJ, Baan J, Jr, Koch KT, DeVries JH, et al. In patients with ST-segment elevation myocardial infarction with cardiogenic shock treated with percutaneous coronary intervention, admission glucose level is a strong independent predictor for 1-year mortality in patients without a prior diagnosis of diabetes. Am Heart J. 2007;154(6):1184–1190. doi: 10.1016/j.ahj.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Kim TH, Yoon KH, Chung WS, Ahn Y, Jeong MH, et al. The stress hyperglycemia ratio, an index of relative hyperglycemia, as a predictor of clinical outcomes after percutaneous coronary intervention. Int J Cardiol. 2017;241:57–63. doi: 10.1016/j.ijcard.2017.02.065. [DOI] [PubMed] [Google Scholar]
- 20.Kattel S, Kasai T, Matsumoto H, Yatsu S, Murata A, Kato T, et al. Association between elevated blood glucose level on admission and long-term mortality in patients with acute decompensated heart failure. J Cardiol. 2017;69(4):619–624. doi: 10.1016/j.jjcc.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Sud M, Wang X, Austin PC, Lipscombe LL, Newton GE, Tu JV, et al. Presentation blood glucose and death, hospitalization, and future diabetes risk in patients with acute heart failure syndromes. Eur Heart J. 2015;36(15):924–931. doi: 10.1093/eurheartj/ehu462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdin A, Pöss J, Fuernau G, Ouarrak T, Desch S, Eitel I, et al. Revision: prognostic impact of baseline glucose levels in acute myocardial infarction complicated by cardiogenic shock-a substudy of the IABP-SHOCK II-trial. Clin Res Cardiol. 2018;107(6):517–523. doi: 10.1007/s00392-018-1213-7. [DOI] [PubMed] [Google Scholar]
- 23.Cohen G, Riahi Y, Alpert E, Gruzman A, Sasson S. The roles of hyperglycaemia and oxidative stress in the rise and collapse of the natural protective mechanism against vascular endothelial cell dysfunction in diabetes. Arch Physiol Biochem. 2007;113(4-5):259–267. doi: 10.1080/13813450701783513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of the patient by the different admission BG levels