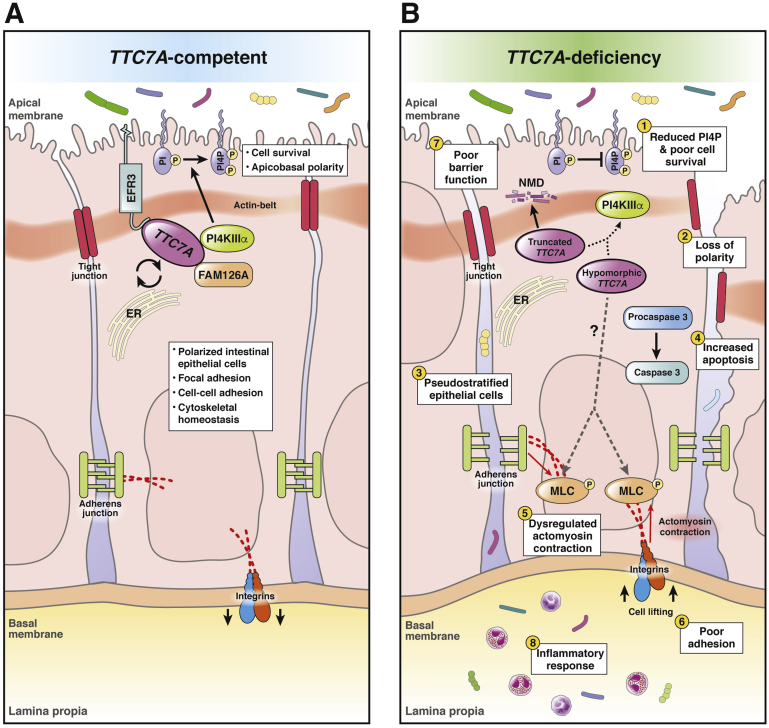
Figure 1.
Summary of the role of TTC7A in intestinal epithelial cells and the consequences of its mutation. (A) TTC7A competent. TTC7A and FAM126A chaperone PI4KIIIα to the plasma membrane.19 EFR3 tethers the PI4KIIIα complex to the plasma membrane where PI4KIIIα catalyzes the conversion of PI to PI4P.18, 19, 24 The synthesis of PI4P is an important regulatory step in the phosphatidyl inositol–phosphate pathway, which is important for survival pathways and intestinal epithelial cell polarity.30 Phosphatidyl inositol–phosphate lipids are differentially produced and turned over on apical and basal membranes of polarized intestinal epithelial cells; thus, phosphatidyl inositol–phosphate levels aid in coordinating apicobasal polarity, which is critical for epithelium integrity.75 Cytoskeletal homeostasis is important in maintaining cell polarity and cell adhesions.5, 35 Tight junctions, adherens junctions, and integrins are regulated in part by actin dynamics and maintain cell adhesions, contributing to intestinal epithelial barrier function.36, 76 (B) TTC7A deficiency. Truncating mutations (ie, nonsense, frameshifts, large deletions) are associated with more severe phenotypes and are predicted to cause a complete loss of function in which TTC7A transcripts are thought to be degraded via nonsense-mediated decay.2, 3, 6, 7, 24 Because TTC7A is a scaffold for multiple proteins, its structure is critical to its role in binding, shuttling, and tethering the PI4KIIIα complex to the plasma membrane; thus, partial or total loss of TTC7A reduces PI4P synthesis (1) via the PI4KIIIα complex (dottedarrow).3, 19 Alterations to the structure of TTC7A (ie, truncated or hypomorphic protein) have been shown to alter the kinase activity and/or the positioning of PI4KIIIα at the membrane.24 Decreases in PI4P affects cell polarity (2), signaling, homeostasis, and survival.30 H&E staining of intestinal biopsy specimens from TTC7A-deficiency patients showed pseudostratification, which points to defects in apicobasal polarity (3). Similarly, tightly regulated apical and basal membranes, marked by the actin belt and integrins, respectively, were mislocalized in TTC7A patient-derived organoids.5, 6 Loss of apicobasal polarity jeopardizes the integrity of the epithelium, contributing to the diverse epithelial defects observed in TTC7A deficiency.5, 6 (4) An increase in intestinal epithelial cell apoptosis, activated via caspase cleavage, was a common feature in patient histology and in vitro functional studies.3, 5, 6, 8 TTC7A deficiency, through unknown mechanisms (dashed arrow), is correlated with increased phosphorylation of myosin light chain (5) and numerous other downstream targets in the RhoA pathway (omitted for simplicity).5, 6 Myosin light-chain phosphorylation initiates actomyosin contraction, which can disturb junction and integrin adhesion proteins and promote cell lifting (6).35, 36, 77, 78 Increases in intestinal epithelial cell apoptosis, actomyosin contraction, and cell lifting disrupts epithelial barrier function (7).54, 56 Furthermore, a weakened intestinal barrier results in the translocation of luminal bacteria into the lamina propria, which can trigger an inflammatory response (8).34, 76 The role of TTC7A in the cell is incompletely understood, making it difficult to link its known functions to the development of MIA, another poorly understood disease.