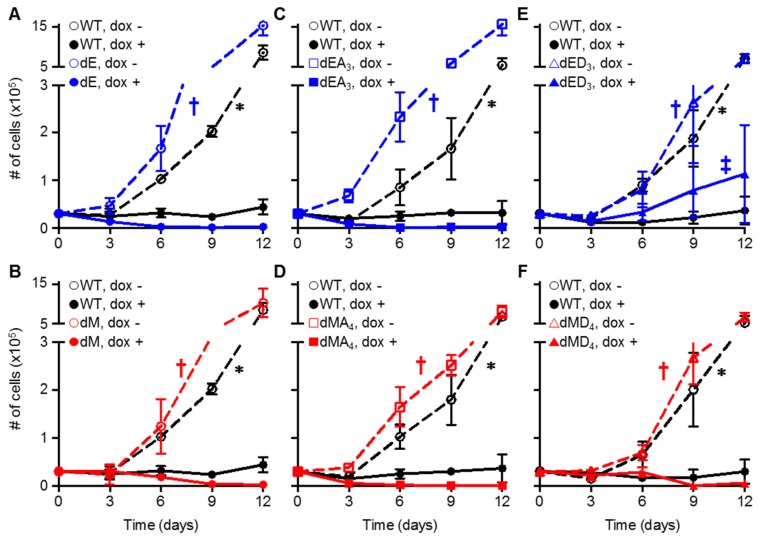
Figure 1.
Both the end-tail and mid-tail regions of the Cx37-CT are necessary and mimicking phosphorylation at S275, S285, and S302 in the Cx37-dE mutant is sufficient for cell survival. Proliferation assays revealed that expression of Cx37-WT (black) initiated death of some cells (days 1–3) and an extended period of growth arrest (days 4–12) of the remaining cells following induced expression (dox +) on day 0. Exponential proliferation was evident in non-expressing (dox -) Rin cells. However, expression of Cx37 with deletions of either the end-tail (dE, blue) or mid-tail (dM, red) alone (A,B), or in combination with alanine substitutions at the remaining putative phosphorylation sites (C,D; dEA3 and dMA4), resulted in death of most, if not all, cells. (E) Aspartate substitution at S275, S285, and S302 with an end-tail deletion (dED3) greatly reduced Cx37-dependent cell death and shortened the growth arrest period such that cells began to slowly proliferate after three days of induced expression. Cx37-dED3 cell cycle time between days 6–12: dox -, 1.93 days; dox +, 3.36 days. (F) Aspartate for serine substitution at 319, 321, 325, and 328 with mid-tail deletion (dMD4) retained the death-inducing properties of Cx37-dM. After 12 days of induced expression, the number of iRin37-dED3 cells was significantly different than the number of -dE and -dEA3 cells. There was no difference in the number of iRin37-dM, -dMA4, and -dMD4 cells. n = 3 in triplicate for all Cx37-isoforms. All values are mean ± s.e.m (where error bars are not evident, they are smaller than the symbol size). ‡ indicates p < 0.05 Cx37-dE versus -dED3, non-parametric ANOVA and Kruskal-Wallis multiple comparisons test. p < 0.05 for dox + versus dox − for all mutants (†), as well as WT (∗).