Abstract
Background: Ischemia-Reperfusion (I/R) injury is the tissue damage that results from re-oxygenation of ischemic tissues. There are many players that contribute to I/R injury. One of these factors is the family of microRNAs (miRNAs), which are currently being heavily studied. This review aims to critically summarize the latest papers that attributed roles of certain miRNAs in I/R injury, particularly in diabetic conditions and dissect their potential as novel pharmacologic targets in the treatment and management of diabetes. Methods: PubMed was searched for publications containing microRNA and I/R, in the absence or presence of diabetes. All papers that provided sufficient evidence linking miRNA with I/R, especially in the context of diabetes, were selected. Several miRNAs are found to be either pro-apoptotic, as in the case of miR-34a, miR-144, miR-155, and miR-200, or anti-apoptotic, as in the case of miR-210, miR-21, and miR-146a. Here, we further dissect the evidence that shows diverse cell-context dependent effects of these miRNAs, particularly in cardiomyocytes, endothelial, or leukocytes. We also provide insight into cases where the possibility of having two miRNAs working together to intensify a given response is noted. Conclusions: This review arrives at the conclusion that the utilization of miRNAs as translational agents or pharmaco-targets in treating I/R injury in diabetic patients is promising and becoming increasingly clearer.
Keywords: pharmaco-targets, diabetes, ischemia-reperfusion injury, microRNA, reactive oxygen species, apoptosis
1. Introduction
Ischemia-Reperfusion (I/R) injury remains a major contributor to two leading causes of death worldwide, ischemic heart disease and stroke [1]. I/R injury results from re-oxygenating tissues that had been deprived of sufficient oxygen (O2) [2]. The hypoxic state that arises due to ischemic conditions renders tissues more sensitive to O2 once circulation is restored. O2 stimulates endothelial cells (ECs), which consequentially produce less nitric oxide (NO) than is needed for cardiovascular homeostasis, thus increasing levels of reactive oxygen species (ROS) [3,4]. ROS are O2-containing free radicals which can precipitate necrosis or apoptosis by damaging cellular DNA, proteins, and lipids [5]. Moreover, the O2-stimulated ECs lose some of their structural integrity, allowing leukocytes to extravasate and start a tissue damage-enticed inflammatory response, accompanied by increased ROS, thus aggravating the I/R injury [6].
Significantly, the risk for stroke and myocardial infarction (MI) and the ensuing I/R injury is particularly high in diabetic patients [7,8]. Diabetes mellitus (DM) is the seventh leading cause of global death [9] with more than 400 million cases of diabetes estimated in 2017 [10]. DM is a chronic disease that is either due to decreased production of insulin, or increased insulin resistance; the former is referred to as type 1 DM (T1DM), and the latter is categorized as type 2 DM (T2DM). Diabetic patients suffer from prolonged hyperglycemia and periods of glucose fluctuations, which have adverse effects on the body, such as the worsening of an I/R injury. The mechanisms through which DM influences I/R injury are largely variable. However, the role of microRNAs (miRNAs) in mediating the link between DM and I/R injury, particularly as pertains to the cardiovasculature, is receiving increased attention but remains far from being fully understood. This review responds to a much-desired need for reviewing and discussing the current literature on such a role.
2. MicroRNAs (miRNAs)
MicroRNAs are noncoding, single-stranded RNA, ranging from 20 to 22 nucleotides in length. Their biogenesis starts when primary miRNAs (pri-mRNAs) are synthesized in the nucleus [11]. These double-stranded pri-mRNAs are cleaved to shorter hairpin RNAs called pre-miRNAs [12]. Pre-miRNAs are further cleaved to shorter double-stranded miRNAs, which then associate with the Argonaute family of proteins to form RNA-induced silencing complexes or RISC. It is in these complexes that mature miRNAs, now single-stranded, is retained [13,14]. MiRNAs play important roles in regulating gene expression by acting as a silencer of messenger RNA (mRNA). By binding to it, miRNA causes the destabilization or cleavage of pseudo-complementary sequences of target mRNA, thus inhibiting its translation [15]. This mechanism was shown to be implicated in many cellular processes such as cell metabolism, division, differentiation, apoptosis, and autophagy [16].
The interest in miRNA as a regulator of many diseases has gained momentum, especially in the cardiovascular field. Extensive research is being conducted to investigate the association of miRNA with CVD and DM. Indeed, the involvement of miRNA, both in detrimental cascades and as potential therapy options affecting angiogenesis, atherosclerosis, hypertension, and ischemic hypoxia, has advanced to the frontline of cardiovascular research [17,18]. Several mechanisms are implicated in the alteration of miRNA expression in diabetes leading to vascular complications including epigenetic DNA-methylation mechanisms affecting miRNA transcription, splicing of intronic miRNA, and disruption of transcription factor sites [19]. In this review, we aim to highlight the role of multiple miRNAs in ROS regulatory pathways in I/R injury, taking the impact of DM into account (Table 1).
Table 1.
A list of miRNAs implicated in diabetes that potentially modulate I/R injury via various mechanisms.
| miRNA | Function | Level in Diabetes |
|---|---|---|
| miR-34 |
|
increased |
| miR-144 |
|
decreased |
| miR-210 |
|
increased |
| miR-141 |
|
increased |
| miR-155 |
|
increased |
| miR-21 |
|
increased |
| miR-146 |
|
increased |
| miR-200b |
|
decreased |
| miR-200c |
|
increased |
2.1. MiR-34a
Recent studies showed that 316 miRNAs had their expression dysregulated by diabetes in a streptozotocin-induced T1DM mouse model. Among these dysregulated miRNAs is miR34a, which is known to play a role in apoptosis, autophagy, and oxidative stress [20,21,22] (Table 1). MiR-34a is reported to silence the mRNA of silent mating type information regulation 2 homolog-1 (sirtuin-1) [23]. Sirtuin-1 is a deacetylase that acts on numerous proteins, thus regulating several cellular functions, including apoptosis (Figure 1) [24]. Interestingly, sirtuin-1 protects cardiomyocytes from I/R-induced apoptosis [25,26,27], whereas cardiomyocyte-specific deletion of Sirt1 renders the myocardium critically sensitive to I/R injury [28].
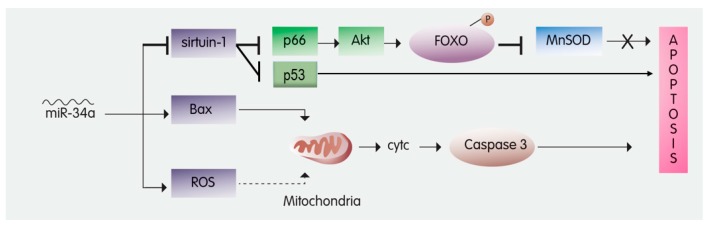
Figure 1.
The role of miR-34a in inducing apoptosis—miR-34a induces apoptosis by inhibiting Sirtuin-1. Sirtuin-1 attenuates apoptosis by: 1—Inhibiting p66, an activator of Akt; Akt phosphorylates FOXO to inhibit MnSOD, which usually inhibits apoptosis. 2—inhibiting the transcription of p53, which usually induces apoptosis. In addition, miR-34a leads to Bax upregulation and reactive oxygen species (ROS) accumulation, hence triggering the release of Cytochrome C from the mitochondria, which activates Caspase 3 to induce apoptosis. FOXO3: Forkhead box O3; MnSOD: Manganese Superoxide Dismutase; Akt: protein kinase B; cytc: Cytochrome c.
There are at least two modes of action through which sirtuin-1 downregulates I/R-induced apoptosis. The first pathway is by inhibiting adaptor protein p66 [29], hence activating forkhead box O3a (FOXO3a). FOXO3a is a transcription factor of manganese superoxide dismutase (MnSOD), a ROS scavenging enzyme (Figure 1) [30]. Alternatively, sirtuin-1 may inhibit the transcriptional activity of p53, thus attenuating apoptosis (Figure 1) [31,32].
Due to its inhibitory effect on sirtuin-1, miR-34a exacerbates myocardial injury by increasing infarct size and promoting apoptosis [26] (Table 1). Importantly, downregulating miR-34a was shown to attenuate I/R injury by inhibiting apoptosis [33]. Indeed, delivering a miR-34a mimic to neonatal hearts significantly inhibited proliferation of cardiomyocytes following MI [34]. Furthermore, blocking miR-34a improved post-MI remodeling, likely via modulating levels of Sirt1, Cyclin D1, and Bcl2 [34]. In DM, miR-34a levels increase by up to five-fold, yet insulin treatment fails to attenuate this dramatic increase miR-34a. [29,35]. Thus, it would not be surprising if DM exacerbates I/R injury due to high miR-34a levels. As such, one can envision that approaches which seek to reduce miR-34a could be used to attenuate I/R injury, as recently suggested [36].
The role of miR-34a in senescence and autophagy is becoming increasingly noted. In senescent endothelial cells, as well as in organs of aged mice, miR-34a appears to be upregulated [37,38,39] (Table 1). Equally importantly, overexpressing miR-34a is sufficient to induce senescence of cells that play an important role in I/R injury, such as endothelial cells or pro-angiogenic cultured progenitor cells [40]. It appears that the autophagy target of miR34 is Atg9 [41]. Very recently, direct evidence for Atg9 in mitochondrial health and cardiac function was established [42]. As mentioned above, levels of miR34 are dramatically increased in DM, with insulin failing to reduce this augmented expression [29,35]. Thus, by increasing miR34, DM may increase autophagic pathways that could contribute to or precipitate I/R injury.
2.2. MiR-144
The role of miR-144 in I/R injury is poorly investigated. However, a recent study suggested a possible role of miR-144 in alleviating diabetic oxidative stress. This study showed that a decrease in miR-144 expression was observed in diabetic cardiomyocytes [43] (Table 1). A similar decrease was also noted in I/R injury in mouse myocardium [44].
MiR-144 appears to aggravate high glucose-induced ROS formation, and this effect is reversed by a Nrf2 activator. Unexpectedly, the expression of Nrf2 was found to be lower in diabetic mice; however, anti-miR144 can reverse this diabetes-induced downregulation of Nrf2 [43]. This is consistent with very recent findings showing that suppressing miR-144 mitigates oxygen-glucose I/R-induced injury via increasing Nrf2 signaling [45].
Although miR-144’s expression was shown to decline in diabetic cardiomyocytes, in vitro experiments showed that the impact of miR-144 on Nrf2 expression was only observed in hyperglycemic conditions, thus reconciling the observations in tissues isolated from T1DM mice [43]. This could potentially implicate other factors being involved in the interplay between miR-144 and Nrf2 expression. Possibly, the downregulation of miR-144 in diabetic cardiomyocytes can be explained as a cellular attempt to respond to oxidative stress by increasing ROS scavengers, albeit to no avail. Significantly, the application of anti-miR-144 was able to decrease ROS levels and attenuate apoptosis following an increase in Nrf2 expression [43]. Henceforth, it may be assumed that the suppression of Nrf2 in DM aids in ROS accumulation and apoptosis in I/R injury. Yet, the exact role and trigger of fluctuations in miR-144 levels remain vague. That said, it is important to note that anti-miR-144 successfully inhibited apoptosis and may thus have a therapeutic potential [43].
Our understanding of the role of miR-144 in autophagy as it pertains to I/R is still in its infancy. It appears that miR-144 can induce pro-autophagic pathways that play a role in cardiac remodeling [44]. This notion is especially noticed when a decrease in miR-144 levels was a key contributor to post-MI maladaptive remodeling [46]. Indeed, this pro-autophagic effect of miR-144 has been shown to improve cardiomyocyte survival [47] and promote cardio-protection [46] (Table 1).
2.3. MiR-210
MiR-210 is a silencer of protein tyrosine phosphatase-1B (Ptp1b) [48], which is an activator of both caspase-8 and caspase-3 [49]. In this sense, miR-210 may elicit an anti-apoptotic effect in response to I/R-induced apoptosis. This is supported by the fact that miR-210 was upregulated in response to H2O2, as an oxidative stressor, in H9c2 rat cardiomyocytes [50]. Other studies lend support to the notion that miR-210 diminishes cardiomyocyte apoptosis induced by oxidative stress [51]. A very recent study reported that the expression of miR-210 is dramatically increased in hypoxic H9c2 cells [52]. Yet this study, in an apparently paradoxical result, reports that when miR-210 is suppressed, hypoxia-induced cardiomyocyte injury was favorably mitigated [52] (Table 1). Regulation of miR-210 expression in response to insulin in the context of I/R injury has also been investigated. Insulin induced the upregulation of miR-210 in H2O2-treated cardiomyoblasts via the PI3K/Akt pathway [50]. Thus, it could be inferred that in DM conditions where low levels of insulin are present, miR-210 is not upregulated, and thus the I/R injury would be more severe.
In marked contrast to the above-mentioned hypothesis, a study showed that in the absence of insulin, miR-210 increased three-fold in diabetic murine hearts [35]. A possible explanation to the increased miR-210 is the accumulation of ROS in diabetic cardiomyocytes. These cells increased their expression of miR-210 as a protective mechanism against ROS-induced apoptosis. This is supported by other studies showing an increase in miR-210 expression in response to H2O2 [50]. It is worth mentioning, however, that despite the upregulation of miR-210, I/R oxidative stress remains more critical in diabetic patients [53,54]. This suggests that caspase-8 and caspase-3 may still be activated by Ptb1b, in addition to other molecules, all of which are upregulated in cases of DM.
Overexpression of miR-210 is regarded as a reliable marker of hypoxia [55,56]. In I/R injury models, levels of this miRNA are significantly increased [57]. Autophagy is also reported in I/R injury [58,59,60]. However, whether miR-210 contributes to this autophagy remains undetermined. It is important to mention that a role for miR-210 in promoting autophagy has been suggested in endometriotic cells [61] (Table 1). Whether this is also the case in myocardial tissue, remains to be established.
2.4. MiR-141
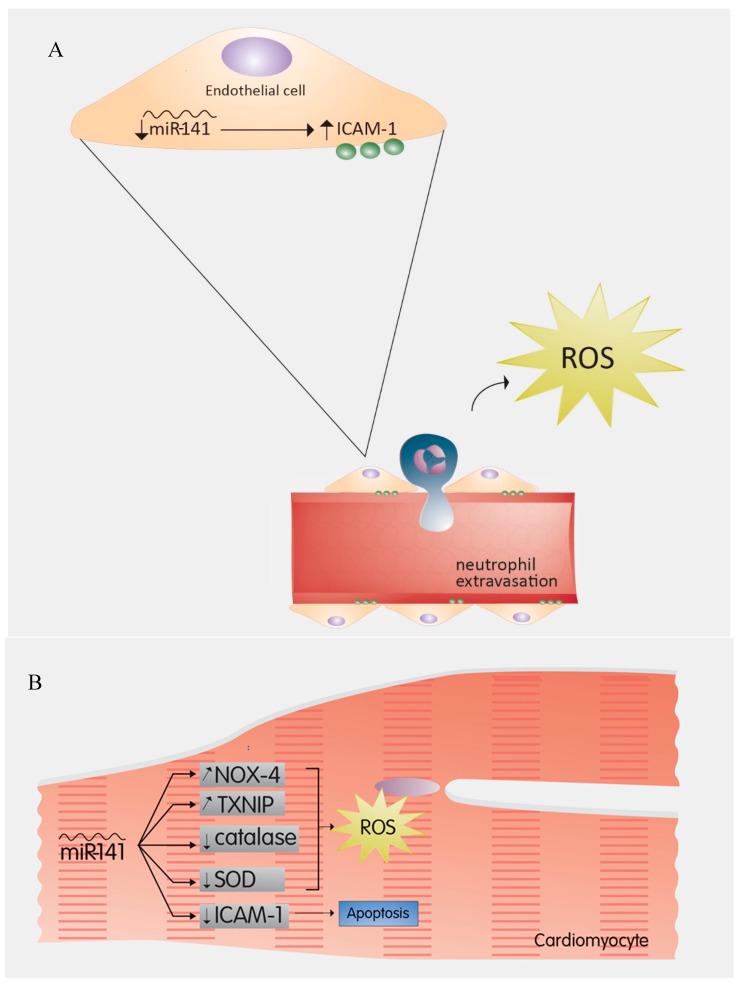
Endothelial cells of cardiac vessels are known to be more prone to apoptosis than cardiomyocytes during I/R injury [62,63,64]. The role of miRNAs in these cells especially under I/R injury conditions is beginning to unravel. In a recent study, miR-141 was shown to play a critical role in the onset of I/R injury by regulating the expression of endothelial intercellular adhesion molecule 1 (ICAM-1) [65]. Treatment of endothelial cells with TNF-α, an ischemia-induced cytokine that promotes the reperfusion injury [66,67,68,69], leads to miR-141 downregulation and ICAM-1 upregulation [65] (Table 1). Administration of exogenous miR-141, however, decreased the expression of endothelial ICAM-1, and subsequently attenuated myocardial injury [65]. This may imply that during I/R injury, the downregulation of miR-141 and the subsequent upregulation of ICAM-1 results in an increased extravasation of neutrophils (Figure 2A). Upon degranulation, neutrophils release ROS and amplify necrosis [70]. This highlights a potential mechanism by which miR-141 imparts protection on ECs in I/R injury.
Figure 2.
(A) miR-141 in endothelial cells: The downregulation of miR-141 and the subsequent upregulation of ICAM-1 results in increased extravasation of neutrophils, which release ROS and amplify necrosis. Hence miR-141 protects endothelial cells in Ischemia-Reperfusion injury. (B) Effects of miR-141 on cardiomyocytes: In cardiomyocytes, the upregulation of miR-141 leads to an increase in ROS by increasing the expression of ROS-generating agents such as NOX-4 and TXNIP, as well as decreasing the activity of antioxidants such as catalase and SOD. In addition, miR-141 silences ICAM-1 expression, hence the induction of apoptosis. ICAM-1: Intercellular Adhesion Molecule 1; NOX-4: NADPH Oxidase 4; TXNIP: Thioredoxin-Interacting Protein; SOD: Superoxide Dismutase.
In the case of DM, miR-141 is upregulated in rat hearts subjugated to glucose fluctuations. Continuous hyperglycemia, like that experienced by hearts of diabetic animals, also evoked a higher expression of miR-141 than normal glucose levels [71]. While this study underscored a beneficial role of miR-141 in the context of non-diabetic I/R, it also showed that the size of the I/R injury was greater in DM hearts compared to controls [71]. The infarct was further amplified when DM hearts were subjected to glucose fluctuations with increasing levels of miR-141 [71]. Such increased levels of miR-141 could promote an increase in the expression of ROS-generating agents, such as NADPH oxidase 4 (NOX4) and thioredoxin-interacting protein (TXNIP), along with a decrease in antioxidant activities of catalase and SOD [71]. The mechanism underlying the amplification of miR-141 and the subsequent effect on ROS-generating enzymes and antioxidants is still unclear but is very likely linked to the cellular O2 burst following blood flow restoration to the ischemic tissue.
The opposing effects that miR-141 has on cardiomyocytes (Figure 2B) when compared to endothelial cells could be due to the notion that miR-141 effect is cell-type dependent with different functions on cardiomyocytes and ECs. Alternatively, it could also mean that despite the fact that miR-141 silences ICAM-1 expression in both cell types, the outcome of such silencing is different and could vary with the presence of comorbidities such as DM. As such, the loss of ICAM-1-mediated cell–cell interaction between cardiomyocytes may underlie the detrimental effects of miR-141 on the myocardium. This is supported by the fact that the loss of ICAM-1-mediated interactions between a cell and its nearby environment promotes cell apoptosis [72].
While miR-141 remains an elusive molecule that deserves more in-depth investigation, the two aforementioned papers draw our attention to the necessity of taking the myocardium as a whole into consideration, rather than just conducting experiments on cardiomyocytes only. Although a miRNA may prove beneficial to one type of cells, it may still be harmful to other cells within the same tissue [73], which would hinder its possible therapeutic use. This is especially important in light of recent findings suggesting a role for miR-141 in promoting [74] or inhibiting [75,76,77] autophagy (Table 1).
2.5. MiR-155
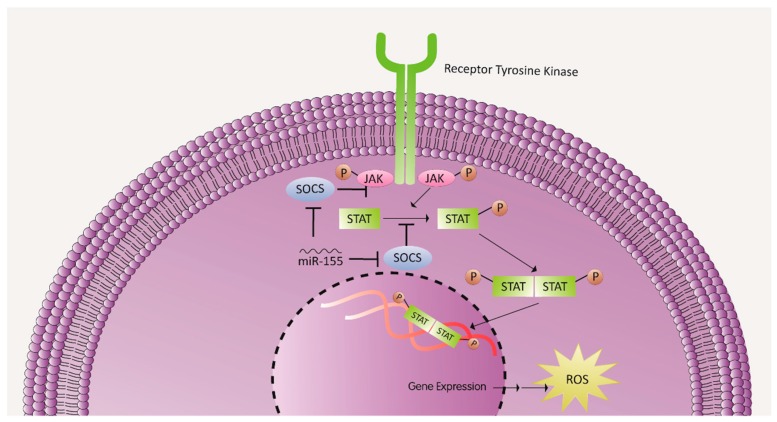
MiRNAs have the potential to affect the heart during I/R injury independent of their cell type. For instance, miR-155, which was shown to increase ROS generation under ischemia-induced myocardial hypoxic conditions, is expressed by infiltrating leukocytes [78]. miR-155 upregulation correlated with an increased activation of macrophages, which lead to the overproduction of inflammatory cytokines and chemoattractants, such as interleukin-1β (IL-1β), TNF-α, and monocyte chemoattractant protein 1 (MCP-1) that further recruited more leukocytes to the ischemic heart [78]. In addition, ROS levels in the recruited inflammatory cells following myocardial ischemia were significantly higher than in miR-155-knockout inflammatory cells [78] (Table 1). Mechanistically, this could suggest that miR-155 increases ROS production by silencing suppressor of cytokine signaling 1 (SOCS-1), a negative regulator of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway which is implicated in ROS generation [78] (Figure 3). This hypothesis is supported by two notions. First, there is a notable complementarity between 3’ UTR of the SOCS-1 transcript and miR-155 [79], and second, silencing SOCS-1 or overexpressing miR-155 resulted in similar effects [78]. However, miR-155 may be acting on other upstream or downstream transcripts with higher affinity, and the JAK/STAT pathway may not be the sole signaling pathway affected by miR-155 overexpression.
Figure 3.
miR-155 role in increased ROS production: miR-155 increases ROS production by silencing SOCS, a negative regulator of the JAK/STAT signaling pathway, a pathway known to play a role in ROS generation. JAK: Janus kinase; STAT: signal transducer and activator of transcription. SOCS: suppressor of cytokine signaling.
In DM, miR-155 is known to be upregulated [80]. Additionally, inhibiting miR-155 is known to reduce cell apoptosis as well as restore cardiac function in a diabetic mouse model [81]. Compromised wound healing that is notable in diabetes is also improved when miR-155 levels are suppressed [82]. Although not studied in the context of diabetic I/R injury, miR-155 inhibition protects against cardiac fibrosis in the diabetic MI mice [83]. Other studies confirmed the pro-fibrotic effect of miR-155 in an MI mouse model [84] or angiotensin II-induced cardiac remodeling [85].
In the context of endothelium-dependent vascular relaxation, it was demonstrated that endothelial nitric oxide synthase (eNOS) is a direct target of miR-155 [86]. NO levels impairment and subsequent endothelial dysfunction substantially increased with miR-155 overexpression [86]. Multiple studies linked miR-155 to the progression of atherosclerosis through promoting macrophage-derived foam cells formation, while others showed an M2 polarization protective profile in the miR-155 KO model of viral myocarditis [87,88,89]. All these findings reveal a multifactorial but harmful effect of miR-155 on the cardiovascular system, especially under I/R injury conditions. Although targeting miR-155 might seem a plausible therapeutic strategy, one must consider the multicellular impact of this molecule. Multiple controversial studies have already emerged highlighting the potential protective impact of miR-155 on the cardiovascular system [90]. These include findings suggesting that decreased expression of miR-155 attenuates oxidant-induced injury and upregulates autophagy via modulating ATG5 in endothelial cells [91] (Table 1).
2.6. MiR-21 and MiR-146a
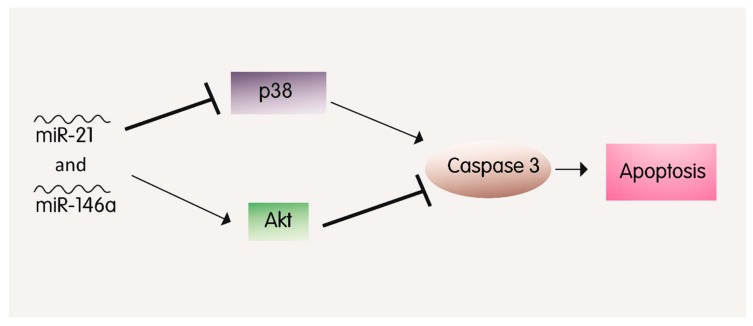
Both miR-21 and miR-146a showed independently exhibit a protective role against hypoxia-induced myocardial apoptosis and inflammation in the context of I/R injury [92,93,94,95]. Exogenous miR-146a has been recently shown to be beneficial in promoting the therapeutic potential of stem cells in the I/R-injured heart [96] (Table 1). Consistently, inhibiting miR-146a caused a dramatic deterioration of cerebral I/R injury [97]. Interestingly, the overexpression of both miR-21 and miR-146a in a MI mouse model attenuated apoptosis of cardiomyocytes to a much higher level than the overexpression of each miRNA individually [57]. This synergistic effect might be due to the fact that each of the aforementioned miRNAs inhibits p38-caspase3-induced apoptosis via different mechanisms [98,99] (Figure 4). For instance, miR-21 attenuates cardiomyocyte injury via regulating the programmed cell death 4 (PDCD4) and AKT pathway, whereas miR-146a exerts its protective antiapoptotic effects through modulating interleukin-1 receptor-associated kinase1 (IRAK1), tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) and the NF-κB/TNF-α pathways [100,101,102]. This finding opens the window to a potential synergistic effect of multiple miRNAs and their impact on disease prevention or progression [57].
Figure 4.
The synergistic role of miR-21 and miR-146a in apoptosis attenuation: miR-21 and miR-146a downregulate p38 and repress Akt inhibition, consequently leading to a decrease in Caspase 3 expression and hence, decreased apoptosis.
In the context of diabetes, both miRNAs are shown to be upregulated under hyperglycemic conditions [103,104,105]. In a myocardial I/R diabetic rat model, miR-21 knockdown exacerbated myocardial apoptosis, thus attenuating the post-conditioning protective effects [106]. Contextually, hydrogen sulfide (H2S) protective effects in the context of diabetic myocardial IR were attributed to the upregulation of miR-21 [107]. MiR-146a, on the other hand, has not been studied in the setting of diabetic myocardial I/R, but multiple studies showed a direct modulatory effect of miR146a on the extracellular matrix, apoptosis, oxidative stress, and inflammatory markers expression in the presence or absence of diabetes [108,109,110,111,112]. For instance, it has been reported that in endothelial cells, fibronectin upregulation by glucose is mediated by miR-146a [109]. Similarly, in T2DM rats, increased expression of miR-146a was correlated with decreased inflammation and reduced oxidative stress status [110].
MiR-146 plays an important role in senescence of many cell types. For instance, expression of miR-146a/b or miR-146a is increased in senescent fibroblasts [113] and endothelial cells [114] respectively (Table 1). This increased expression of miR-146a in aortic endothelial cells appears to be associated with a senescent secretory phenotype [114]. Importantly, by virtue of its ability to regulate IRAK in the heart, miR-146a can protect against immune-hyperresponsiveness during I/R injury [115]. Although a role in autophagy has not been reported for miR-21, it appears to protect against cardiac I/R injury by its ability to inhibit excessively augmented autophagic pathways [116]. Consistent with the potential synergy between miR-146 and miR-21, it was recently reported that upregulation of miR-21 is associated with decreased autophagy in myocardial tissues [117].
Taken together, both miR-21 and miR-146a seem therapeutically promising within the context of diabetic I/R. However, one must not overlook their pro-survival effects that could elicit, for example, an adverse fibrotic response or a worsened diabetic retinopathy [108,118,119,120,121]. Indeed, this becomes important since it was very recently shown that downregulating miR-146a-5p may be needed in alleviating I/R injury [122]. Therefore, additional research is still required to better elaborate on the effects of individual and synergetic application of these miRNAs.
2.7. MiR-200c
Mir-200 is another well-known family of miRNAs but poorly investigated in cardiovascular diseases. It consists of five members known as miR-200a, -200b, -200c,-141, and -429. Multiple studies recently emerged linking miR-200 to the adverse outcomes of DM, obesity, atherosclerosis, and vascular dysfunction [123,124,125]. Magenta et al. were the first to report that miR-200c expression in endothelial and smooth muscle cells was ROS-mediated [126]. MiR-200c-mediated vascular dysfunction has been linked to its ability to decrease ROS scavengers, increase ROS production, and decrease NO formation [127] (Table 1). Additionally, in the setting of diabetes, miR-200c was directly linked to pro-inflammatory responses and apoptosis of vascular smooth muscle cells (VSMCs) and endothelial cells, respectively [126,128]. This was confirmed by using miR-200c inhibitors that improved endothelium-dependent vasorelaxation of diabetic aortas [129].
Although miR-200c is upregulated in the context of ischemia (skeletal muscle) and I/R (brain), its role in myocardial I/R in the presence or absence of diabetes is still largely unknown [130]. However, its levels in diabetics are higher than in control mice [124] (Table 1). In a recent study, miR-200c was linked to enhanced myocardial I/R injury in diabetes, possibly through ROS outburst which was further exacerbated with glucose fluctuation [71]. Of note, not all members of the miR-200 family exert adverse effects. For instance, endothelial miR-200b overexpression prevents diastolic dysfunction and endothelial-to-mesenchymal transition, preserving, therefore, cellular integrity and preventing diabetes-induced adverse myocardial structural and functional changes [131] (Table 1). Importantly, very recently, a report was published showing that levels of miR-200b are lower in patients with T2DM than in control ones [132] (Table 1). Further studies are required in order to elaborate on the different protective and/or adverse impacts that each member of the miR-200 family has in a cell and disease dependent manner in order to optimize any potential therapeutic approach.
2.8. Other Promising miRNAs as Therapeutic Targets
Multiple miRNAs are recently gaining more attention in the context of I/R therapy. In a very recent study, miR-24 that was shown to be decreased in the plasma of diabetic humans, reducing cardiac damage following I/R when overexpressed in the heart of a diabetic rat model [71]. The proposed mechanisms behind those findings were linked to the inhibition of apoptosis, autophagy, and O-GlcNAcylation. MiR-24 has been also linked to apoptosis and fibrosis modulation in cardiac remodeling following a MI [133,134]. Mir-126, another promising target that plays a protective role in I/R vascular homeostasis and angiogenesis, is now known to be downregulated in diabetes [135,136]. Jansen et al. recently demonstrated that patients with diabetes that are at highest risk of coronary artery disease have reduced mir-126 and mir-26a packaging into the endothelial microparticles (EMPs) [137]. In accordance with the potential protective effects, Babu et al. showed that miR-126 overexpression promotes appropriate efferocytosis of apoptotic cardiomyocytes that were otherwise impaired with diabetes [138]. Linking the downregulation of miR-126 EMP packaging with diabetes to the important efferocytotic and angiogenic effects of miR-126, one would expect a worsened remodeling of the heart in the context of diabetic I/R. Further investigation however is still warranted. MiR-17 in another miRNA that has been shown to protect against myocardial [139] and renal [140,141] I/R injury. Interestingly, miR-17 appears to promote hepatic I/R injury via suppressing STAT expression [142]. Last but not least, miR-135a overexpression protected myocardial cells from apoptosis by decreasing TXNIP expression in a diabetic mouse model of I/R [143].
Multiple other miRNAs are gaining more attention in the context of IR injury either due to their potential protective or harmful effects. For instance, miR-133,-146b, 199a-3p, -210, -494, and -499 exert anti-apoptotic effects and protect the myocytes against I/R injury, whereas miR-1, -29,-128, -199a-5p, and -320 promote apoptosis post-myocardial I/R [144,145,146,147,148,149,150,151,152] [153,154,155,156]. Additional experiments are required in order to unveil the underlying mechanisms behind those effects and their role in the context of diabetic IR injury
3. Conclusions
In conclusion, the contribution of miRNA to I/R injury in diabetic patients, whether protective or detrimental, is significant. Ostensibly, pro-apoptotic miRNAs are more capable of exercising their effect when compared to the anti-apoptotic ones, most probably due to the diabetic cellular machinery, which is skewed more towards ROS-generating rather than ROS-scavenging. Another probable factor is the presence of extrinsic factors that are upregulating ROS-generating molecules, or downregulating scavengers, diminishing, therefore, the protective effects of miRNAs. Nevertheless, utilization of miRNAs as translational agents or pharmaco-targets in treating I/R injury in diabetic patients could be attractive for future investigations. However, more thorough investigation is needed to elaborate on the effect of specific miRNAs on the system as a whole, rather than on a specific cell type of the studied organ or tissue.
Funding
This publication was made possible by an MPP fund [#320133] from the American University of Beirut-Faculty of Medicine to Ali H. Eid and from the Qatar University Grants [QUCG-CHS-2018\2019-1] and [QUIRCC-CHS-2019\2020] to Gianfranco Pintus. The article processing charge (APC) for the publication of this article was funded by the [Qatar National Library].
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy D.J., Yellon D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagliaro P., Chiribiri A., Mancardi D., Rastaldo R., Gattullo D., Losano G. Coronary endothelial dysfunction after ischemia and reperfusion and its prevention by ischemic preconditioning. Ital. Heart J. 2003;4:383–394. [PubMed] [Google Scholar]
- 4.Folino A., Losano G., Rastaldo R. Balance of nitric oxide and reactive oxygen species in myocardial reperfusion injury and protection. J. Cardiovasc. Pharmacol. 2013;62:567–575. doi: 10.1097/FJC.0b013e3182a50c45. [DOI] [PubMed] [Google Scholar]
- 5.Martindale J.L., Holbrook N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 6.Maroszynska I., Fiedor P. Leukocytes and endothelium interaction as rate limiting step in the inflammatory response and a key factor in the ischemia-reperfusion injury. Ann. Transplant. 2000;5:5–11. [PubMed] [Google Scholar]
- 7.Haffner S.M., Lehto S., Ronnemaa T., Pyorala K., Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 8.Liao C.C., Shih C.C., Yeh C.C., Chang Y.C., Hu C.J., Lin J.G., Chen T.L. Impact of Diabetes on Stroke Risk and Outcomes: Two Nationwide Retrospective Cohort Studies. Medicine (Baltimore) 2015;94:e2282. doi: 10.1097/MD.0000000000002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO The top 10 causes of death. [(accessed on 17 July 2018)];2016 World Health Organization. Available online: http://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 10.IDF Diabetes Atlas Eighth Edition 2017. [(accessed on 12 November 2018)];2017 Available online: http://www.diabetesatlas.org/
- 11.Lee Y., Kim M., Han J., Yeom K.H., Lee S., Baek S.H., Kim V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., Lee J., Provost P., Radmark O., Kim S., et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 13.Hutvagner G., Mclachlan J., Pasquinelli A.E., Balint E., Tuschl T., Zamore P.D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 14.Treiber T., Treiber N., Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell. Biol. 2019;20:5–20. doi: 10.1038/s41580-018-0059-1. [DOI] [PubMed] [Google Scholar]
- 15.Fabian M.R., Sonenberg N., Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 16.Sermersheim M.A., Park K.H., Gumpper K., Adesanya T.M., Song K., Tan T., Ren X., Yang J.M., Zhu H. MicroRNA regulation of autophagy in cardiovascular disease. Front. Biosci. (Landmark Ed.) 2017;22:48–65. doi: 10.2741/4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barwari T., Joshi A., Mayr M. MicroRNAs in Cardiovascular Disease. J. Am. Coll. Cardiol. 2016;68:2577–2584. doi: 10.1016/j.jacc.2016.09.945. [DOI] [PubMed] [Google Scholar]
- 18.De Rosa S., Curcio A., Indolfi C. Emerging role of microRNAs in cardiovascular diseases. Circ. J. 2014;78:567–575. doi: 10.1253/circj.CJ-14-0086. [DOI] [PubMed] [Google Scholar]
- 19.La Sala L., Micheloni S., De Nigris V., Prattichizzo F., Ceriello A. Novel insights into the regulation of miRNA transcriptional control: Implications for T2D and related complications. Acta Diabetol. 2018;55:989–998. doi: 10.1007/s00592-018-1149-4. [DOI] [PubMed] [Google Scholar]
- 20.Kim S.M., Hur D.Y., Hong S.W., Kim J.H. EBV-encoded EBNA1 regulates cell viability by modulating miR34a-NOX2-ROS signaling in gastric cancer cells. Biochem. Biophys. Res. Commun. 2017;494:550–555. doi: 10.1016/j.bbrc.2017.10.095. [DOI] [PubMed] [Google Scholar]
- 21.Huang X., Gao Y., Qin J., Lu S. The mechanism of long non-coding RNA MEG3 for hepatic ischemia-reperfusion: Mediated by miR-34a/Nrf2 signaling pathway. J. Cell Biochem. 2018;119:1163–1172. doi: 10.1002/jcb.26286. [DOI] [PubMed] [Google Scholar]
- 22.Verjans R., Van Bilsen M., Schroen B. MiRNA Deregulation in Cardiac Aging and Associated Disorders. Int. Rev. Cell Mol. Biol. 2017;334:207–263. doi: 10.1016/bs.ircmb.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Yamakuchi M., Ferlito M., Lowenstein C.J. MiR-34a repression of SIRT1 regulates apoptosis. Proc. Natl. Acad. Sci. USA. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michan S., Sinclair D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Y., Zhang L., Li F., Hu C.P., Zhang Z. Restoration of sirt1 function by pterostilbene attenuates hypoxia-reoxygenation injury in cardiomyocytes. Eur. J. Pharmacol. 2016;776:26–33. doi: 10.1016/j.ejphar.2016.02.052. [DOI] [PubMed] [Google Scholar]
- 26.Fu B.C., Lang J.L., Zhang D.Y., Sun L., Chen W., Liu W., Liu K.Y., Ma C.Y., Jiang S.L., Li R.K., et al. Suppression of miR-34a Expression in the Myocardium Protects Against Ischemia-Reperfusion Injury through SIRT1 Protective Pathway. Stem Cells Dev. 2017;26:1270–1282. doi: 10.1089/scd.2017.0062. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y., Feng Y., Liu D., Zhang Z., Gao K., Zhang W., Tang H. Thymoquinone Attenuates Myocardial Ischemia/Reperfusion Injury Through Activation of SIRT1 Signaling. Cell Physiol. Biochem. 2018;47:1193–1206. doi: 10.1159/000490216. [DOI] [PubMed] [Google Scholar]
- 28.Wang L., Quan N., Sun W., Chen X., Cates C., Rousselle T., Zhou X., Zhao X., Li J. Cardiomyocyte-specific deletion of Sirt1 gene sensitizes myocardium to ischaemia and reperfusion injury. Cardiovasc. Res. 2018;114:805–821. doi: 10.1093/cvr/cvy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G., Yao J., Li Z., Zu G., Feng D., Shan W., Li Y., Hu Y., Zhao Y., Tian X. MiR-34a-5p Inhibition Alleviates Intestinal Ischemia/Reperfusion-Induced Reactive Oxygen Species Accumulation and Apoptosis via Activation of SIRT1 Signaling. Antioxid. Redox Signal. 2016;24:961–973. doi: 10.1089/ars.2015.6492. [DOI] [PubMed] [Google Scholar]
- 30.Galimov E.R. The Role of p66shc in Oxidative Stress and Apoptosis. Acta Nat. 2010;2:44–51. [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G., Wang J.J., To T.S., Zhao H.F., Wang J. Role of SIRT1-mediated mitochondrial and Akt pathways in glioblastoma cell death induced by Cotinus coggygria flavonoid nanoliposomes. Int. J. Nanomed. 2015;10:5005–5023. doi: 10.2147/IJN.S82282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamakuchi M., Lowenstein C.J. MiR-34, SIRT1 and p53: The feedback loop. Cell Cycle. 2009;8:712–715. doi: 10.4161/cc.8.5.7753. [DOI] [PubMed] [Google Scholar]
- 33.Xu D.M., Li H., Zhao Y., Wang C.S. Downregulation of miR-34a attenuates myocardial ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis. Int. J. Clin. Exp. Pathol. 2017;10:3865–3875. [Google Scholar]
- 34.Yang Y., Cheng H.W., Qiu Y., Dupee D., Noonan M., Lin Y.D., Fisch S., Unno K., Sereti K.I., Liao R. MicroRNA-34a Plays a Key Role in Cardiac Repair and Regeneration Following Myocardial Infarction. Circ. Res. 2015;117:450–459. doi: 10.1161/CIRCRESAHA.117.305962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costantino S., Paneni F., Luscher T.F., Cosentino F. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur. Heart J. 2016;37:572–576. doi: 10.1093/eurheartj/ehv599. [DOI] [PubMed] [Google Scholar]
- 36.Qin L.B., Li Z.Y., Li H., Fan X.Q., Liu H.G., Dong X.M., Jia W.Y. Inhibitive effects of microRNA-34a on protecting against ischemia-reperfusion injury of vital organs in hemorrhagic shock pregnant mice. Eur. Rev. Med. Pharmacol. Sci. 2018;22:1812–1818. doi: 10.26355/eurrev_201803_14600. [DOI] [PubMed] [Google Scholar]
- 37.Ito T., Yagi S., Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem. Biophys. Res. Commun. 2010;398:735–740. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Menghini R., Casagrande V., Cardellini M., Martelli E., Terrinoni A., Amati F., Vasa-Nicotera M., Ippoliti A., Novelli G., Melino G., et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 39.Li X., Khanna A., Li N., Wang E. Circulatory miR-34a as an RNA-based, noninvasive biomarker for brain aging. Aging (Albany NY) 2011;3:985. doi: 10.18632/aging.100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao T., Li J., Chen A.F. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am. J. Physiol. Endocrinol. Metab. 2010;299:E110–E116. doi: 10.1152/ajpendo.00192.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J., Chen D., He Y., Melendez A., Feng Z., Hong Q., Bai X., Li Q., Cai G., Wang J., et al. MiR-34 modulates Caenorhabditis elegans lifespan via repressing the autophagy gene atg9. Age (Dordr) 2013;35:11–22. doi: 10.1007/s11357-011-9324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu P., Damschroder D., Zhang M., Ryall K.A., Adler P.N., Saucerman J.J., Wessells R.J., Yan Z. Atg2, Atg9 and Atg18 in mitochondrial integrity, cardiac function and healthspan in Drosophila. J. Mol. Cell Cardiol. 2018;127:116–124. doi: 10.1016/j.yjmcc.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu M., Liu Y., Zhang B., Shi Y., Cui L., Zhao X. Inhibiting microRNA-144 abates oxidative stress and reduces apoptosis in hearts of streptozotocin-induced diabetic mice. Cardiovasc. Pathol. 2015;24:375–381. doi: 10.1016/j.carpath.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Li J., Rohailla S., Gelber N., Rutka J., Sabah N., Gladstone R.A., Wei C., Hu P., Kharbanda R.K., Redington A.N. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res. Cardiol. 2014;109:423. doi: 10.1007/s00395-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 45.Li Y., Zhao Y., Cheng M., Qiao Y., Wang Y., Xiong W., Yue W. Suppression of microRNA-144-3p attenuates oxygen-glucose deprivation/reoxygenation-induced neuronal injury by promoting Brg1/Nrf2/ARE signaling. J. Biochem. Mol. Toxicol. 2018;32:e22044. doi: 10.1002/jbt.22044. [DOI] [PubMed] [Google Scholar]
- 46.Li J., Cai S.X., He Q., Zhang H., Friedberg D., Wang F., Redington A.N. Intravenous miR-144 reduces left ventricular remodeling after myocardial infarction. Basic Res. Cardiol. 2018;113:36. doi: 10.1007/s00395-018-0694-x. [DOI] [PubMed] [Google Scholar]
- 47.Aoyagi T., Kusakari Y., Xiao C.Y., Inouye B.T., Takahashi M., Scherrer-Crosbie M., Rosenzweig A., Hara K., Matsui T. Cardiac mTOR protects the heart against ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H75–H85. doi: 10.1152/ajpheart.00241.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barile L., Lionetti V., Cervio E., Matteucci M., Gherghiceanu M., Popescu L.M., Torre T., Siclari F., Moccetti T., Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 49.Song H., Zhang Z., Wang L. Small interference RNA against PTP-1B reduces hypoxia/reoxygenation induced apoptosis of rat cardiomyocytes. Apoptosis. 2008;13:383–393. doi: 10.1007/s10495-008-0181-1. [DOI] [PubMed] [Google Scholar]
- 50.Shi Y.F., Liu N., Li Y.X., Song C.L., Song X.J., Zhao Z., Liu B. Insulin protects H9c2 rat cardiomyoblast cells against hydrogen peroxide-induced injury through upregulation of microRNA-210. Free Radic. Res. 2015;49:1147–1155. doi: 10.3109/10715762.2015.1050588. [DOI] [PubMed] [Google Scholar]
- 51.Diao H., Liu B., Shi Y., Song C., Guo Z., Liu N., Song X., Lu Y., Lin X., Li Z. MicroRNA-210 alleviates oxidative stress-associated cardiomyocyte apoptosis by regulating BNIP3. Biosci. Biotechnol. Biochem. 2017;81:1712–1720. doi: 10.1080/09168451.2017.1343118. [DOI] [PubMed] [Google Scholar]
- 52.Feng M., Li Z., Wang D., Wang F., Wang C., Wang C., Ding F. MicroRNA-210 aggravates hypoxia-induced injury in cardiomyocyte H9c2 cells by targeting CXCR4. Biomed. Pharmacother. 2018;102:981–987. doi: 10.1016/j.biopha.2018.03.151. [DOI] [PubMed] [Google Scholar]
- 53.Mahalakshmi A., Kurian G.A. Evaluating the impact of diabetes and diabetic cardiomyopathy rat heart on the outcome of ischemia-reperfusion associated oxidative stress. Free Radic. Biol. Med. 2018;118:35–43. doi: 10.1016/j.freeradbiomed.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Wu M.Y., Yiang G.T., Lai T.T., Li C.J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxid. Med. Cell Longev. 2018;2018:3420187. doi: 10.1155/2018/3420187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biswas S., Roy S., Banerjee J., Hussain S.R., Khanna S., Meenakshisundaram G., Kuppusamy P., Friedman A., Sen C.K. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc. Natl. Acad. Sci. USA. 2010;107:6976–6981. doi: 10.1073/pnas.1001653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gee H.E., Camps C., Buffa F.M., Patiar S., Winter S.C., Betts G., Homer J., Corbridge R., Cox G., West C.M., et al. hsa-mir-210 is a marker of tumor hypoxia and a prognostic factor in head and neck cancer. Cancer. 2010;116:2148–2158. doi: 10.1002/cncr.25009. [DOI] [PubMed] [Google Scholar]
- 57.Ouyang F., Huang H., Zhang M., Chen M., Huang H., Huang F., Zhou S. HMGB1 induces apoptosis and EMT in association with increased autophagy following H/R injury in cardiomyocytes. Int. J. Mol. Med. 2016;37:679–689. doi: 10.3892/ijmm.2016.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren Z., Xiao W., Zeng Y., Liu M.H., Li G.H., Tang Z.H., Qu S.L., Hao Y.M., Yuan H.Q., Jiang Z.S. Fibroblast growth factor-21 alleviates hypoxia/reoxygenation injury in H9c2 cardiomyocytes by promoting autophagic flux. Int. J. Mol. Med. 2019;43:1321–1330. doi: 10.3892/ijmm.2019.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang X., Lew K.S., Chen Q., Richards A.M., Wang P. Human mesenchymal stem cell-derived exosomes reduce ischemia/reperfusion injury by the inhibitions of apoptosis and autophagy. Curr. Pharm. Des. 2019 doi: 10.2174/1381612825666190119130441. [DOI] [PubMed] [Google Scholar]
- 60.Shi B., Ma M., Zheng Y., Pan Y., Lin X. mTOR and Beclin1: Two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. J. Cell. Physiol. 2019 doi: 10.1002/jcp.28125. [DOI] [PubMed] [Google Scholar]
- 61.Xu T.X., Zhao S.Z., Dong M., Yu X.R. Hypoxia responsive miR-210 promotes cell survival and autophagy of endometriotic cells in hypoxia. Eur. Rev. Med. Pharmacol. Sci. 2016;20:399–406. [PubMed] [Google Scholar]
- 62.Singhal A.K., Symons J.D., Boudina S., Jaishy B., Shiu Y.T. Role of Endothelial Cells in Myocardial Ischemia-Reperfusion Injury. Vasc. Dis. Prev. 2010;7:1–14. doi: 10.2174/1874120701007010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brutsaert D.L. Cardiac endothelial-myocardial signaling: Its role in cardiac growth, contractile performance, and rhythmicity. Physiol. Rev. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- 64.Quintero M., Colombo S.L., Godfrey A., Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc. Natl. Acad. Sci. USA. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu R.R., Li J., Gong J.Y., Kuang F., Liu J.Y., Zhang Y.S., Ma Q.L., Song C.J., Truax A.D., Gao F., et al. MicroRNA-141 regulates the expression level of ICAM-1 on endothelium to decrease myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H1303–H1313. doi: 10.1152/ajpheart.00290.2015. [DOI] [PubMed] [Google Scholar]
- 66.Saini H.K., Xu Y.J., Zhang M., Liu P.P., Kirshenbaum L.A., Dhalla N.S. Role of tumour necrosis factor-alpha and other cytokines in ischemia-reperfusion-induced injury in the heart. Exp. Clin. Cardiol. 2005;10:213–222. [PMC free article] [PubMed] [Google Scholar]
- 67.Maekawa N., Wada H., Kanda T., Niwa T., Yamada Y., Saito K., Fujiwara H., Sekikawa K., Seishima M. Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-alpha. J. Am. Coll. Cardiol. 2002;39:1229–1235. doi: 10.1016/S0735-1097(02)01738-2. [DOI] [PubMed] [Google Scholar]
- 68.Bellisarii F.L., Gallina S., De Caterina R. Tumor necrosis factor-alpha and cardiovascular diseases. Ital. Heart J. 2001;2:408–417. [PubMed] [Google Scholar]
- 69.Dhote-Burger P., Vuilleminot A., Lecompte T., Pasquier C., Bara L., Julia P., Chardigny C., Fabiani J.N. Neutrophil degranulation related to the reperfusion of ischemic human heart during cardiopulmonary bypass. J. Cardiovasc. Pharmacol. 1995;25(Suppl. 2):S124–S129. doi: 10.1097/00005344-199500252-00026. [DOI] [PubMed] [Google Scholar]
- 70.Wang Q., Doerschuk C.M. Neutrophil-induced changes in the biomechanical properties of endothelial cells: Roles of ICAM-1 and reactive oxygen species. J. Immunol. 2000;164:6487–6494. doi: 10.4049/jimmunol.164.12.6487. [DOI] [PubMed] [Google Scholar]
- 71.Saito S., Thuc L.C., Teshima Y., Nakada C., Nishio S., Kondo H., Fukui A., Abe I., Ebata Y., Saikawa T., et al. Glucose Fluctuations Aggravate Cardiac Susceptibility to Ischemia/Reperfusion Injury by Modulating MicroRNAs Expression. Circ. J. 2016;80:186–195. doi: 10.1253/circj.CJ-14-1218. [DOI] [PubMed] [Google Scholar]
- 72.Martinesi M., Treves C., D’albasio G., Bagnoli S., Bonanomi A.G., Stio M. Vitamin D derivatives induce apoptosis and downregulate ICAM-1 levels in peripheral blood mononuclear cells of inflammatory bowel disease patients. Inflamm. Bowel Dis. 2008;14:597–604. doi: 10.1002/ibd.20354. [DOI] [PubMed] [Google Scholar]
- 73.Li X.-Y., Wang S.-S., Han Z., Han F., Chang Y.-P., Yang Y., Xue M., Sun B., Chen L.-M. Triptolide restores autophagy to alleviate diabetic renal fibrosis through the miR-141-3p/PTEN/Akt/mTOR pathway. Mol. Ther. Nucl. Acids. 2017;9:48–56. doi: 10.1016/j.omtn.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu K., Hou Y., Liu Y., Zheng J. LncRNA SNHG15 contributes to proliferation, invasion and autophagy in osteosarcoma cells by sponging miR-141. J. Biomed. Sci. 2017;24:46. doi: 10.1186/s12929-017-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y., Liu Y., Xue J., Yang Z., Shi Y., Shi Y., Lou G., Wu S., Qi J., Liu W., et al. MicroRNA-141 Targets Sirt1 and Inhibits Autophagy to Reduce HBV Replication. Cell. Physiol. Biochem. 2017;41:310–322. doi: 10.1159/000456162. [DOI] [PubMed] [Google Scholar]
- 76.Zhu H., Huang L., Zhu S., Li X., Li Z., Yu C., Yu X. Regulation of autophagy by systemic admission of microrna-141 to target hmgb1 in l-arginine-induced acute pancreatitis in vivo. Pancreatology. 2016;16:337–346. doi: 10.1016/j.pan.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Preusse M., Theis F.J., Mueller N.S. Mitalos v2: Analyzing tissue specific microrna function. PLoS ONE. 2016;11:e0151771. doi: 10.1371/journal.pone.0151771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eisenhardt S.U., Weiss J.B., Smolka C., Maxeiner J., Pankratz F., Bemtgen X., Kustermann M., Thiele J.R., Schmidt Y., Bjoern Stark G., et al. Microrna-155 aggravates ischemia-reperfusion injury by modulation of inflammatory cell recruitment and the respiratory oxidative burst. Basic Res. Cardiol. 2015;110:32. doi: 10.1007/s00395-015-0490-9. [DOI] [PubMed] [Google Scholar]
- 79.Yao R., Ma Y.L., Liang W., Li H.H., Ma Z.J., Yu X., Liao Y.H. Microrna-155 modulates treg and th17 cells differentiation and th17 cell function by targeting socs1. PLoS ONE. 2012;7:e46082. doi: 10.1371/journal.pone.0046082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kwon D.N., Chang B.S., Kim J.H. Microrna dysregulation in liver and pancreas of cmp-neu5ac hydroxylase null mice disrupts insulin/pi3k-akt signaling. Biomed. Res. Int. 2014;2014:236385. doi: 10.1155/2014/236385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jia C., Chen H., Wei M., Chen X., Zhang Y., Cao L., Yuan P., Wang F., Yang G., Ma J. Gold nanoparticle-based mir155 antagonist macrophage delivery restores the cardiac function in ovariectomized diabetic mouse model. Int. J. Nanomed. 2017;12:4963–4979. doi: 10.2147/IJN.S138400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ye J., Kang Y., Sun X., Ni P., Wu M., Lu S. Microrna-155 inhibition promoted wound healing in diabetic rats. Int. J. Low. Extrem. Wounds. 2017;16:74–84. doi: 10.1177/1534734617706636. [DOI] [PubMed] [Google Scholar]
- 83.Kishore R., Verma S.K., Mackie A.R., Vaughan E.E., Abramova T.V., Aiko I., Krishnamurthy P. Bone marrow progenitor cell therapy-mediated paracrine regulation of cardiac mirna-155 modulates fibrotic response in diabetic hearts. PLoS ONE. 2013;8:e60161. doi: 10.1371/journal.pone.0060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He W., Huang H., Xie Q., Wang Z., Fan Y., Kong B., Huang D., Xiao Y. Mir-155 knockout in fibroblasts improves cardiac remodeling by targeting tumor protein p53-inducible nuclear protein 1. J. Cardiovasc. Pharmacol. Ther. 2016;21:423–435. doi: 10.1177/1074248415616188. [DOI] [PubMed] [Google Scholar]
- 85.Wei Y., Yan X., Yan L., Hu F., Ma W., Wang Y., Lu S., Zeng Q., Wang Z. Inhibition of microrna155 ameliorates cardiac fibrosis in the process of angiotensin iiinduced cardiac remodeling. Mol. Med. Rep. 2017;16:7287–7296. doi: 10.3892/mmr.2017.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun H.X., Zeng D.Y., Li R.T., Pang R.P., Yang H., Hu Y.L., Zhang Q., Jiang Y., Huang L.Y., Tang Y.B., et al. Essential role of microrna-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. 2012;60:1407–1414. doi: 10.1161/HYPERTENSIONAHA.112.197301. [DOI] [PubMed] [Google Scholar]
- 87.Wu X.Y., Fan W.D., Fang R., Wu G.F. Regulation of microrna-155 in endothelial inflammation by targeting nuclear factor (nf)-kappab p65. J. Cell. Biochem. 2014;115:1928–1936. doi: 10.1002/jcb.24864. [DOI] [PubMed] [Google Scholar]
- 88.Tian F.J., An L.N., Wang G.K., Zhu J.Q., Li Q., Zhang Y.Y., Zeng A., Zou J., Zhu R.F., Han X.S., et al. Elevated microrna-155 promotes foam cell formation by targeting hbp1 in atherogenesis. Cardiovasc. Res. 2014;103:100–110. doi: 10.1093/cvr/cvu070. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y., Zhang M., Li X., Tang Z., Wang X., Zhong M., Suo Q., Zhang Y., Lv K. Silencing microrna-155 attenuates cardiac injury and dysfunction in viral myocarditis via promotion of M2 phenotype polarization of macrophages. Sci. Rep. 2016;6:22613. doi: 10.1038/srep22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao R.Y., Li Q., Miao Y., Zhang Y., Yuan W., Fan L., Liu G., Mi Q., Yang J. The emerging role of microrna-155 in cardiovascular diseases. Biomed. Res. Int. 2016;2016:9869208. doi: 10.1155/2016/9869208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen H., Gao L., Yang M., Zhang L., He F.L., Shi Y.K., Pan X.H., Wang H. Microrna-155 affects oxidative damage through regulating autophagy in endothelial cells. Oncol. Lett. 2019;17:2237–2243. doi: 10.3892/ol.2018.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheng Y., Zhu P., Yang J., Liu X., Dong S., Wang X., Chun B., Zhuang J., Zhang C. Ischaemic preconditioning-regulated mir-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target pdcd4. Cardiovasc. Res. 2010;87:431–439. doi: 10.1093/cvr/cvq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X., Ha T., Liu L., Zou J., Zhang X., Kalbfleisch J., Gao X., Williams D., Li C. Increased expression of microrna-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2013;97:432–442. doi: 10.1093/cvr/cvs356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qiao S., Olson J.M., Paterson M., Yan Y., Zaja I., Liu Y., Riess M.L., Kersten J.R., Liang M., Warltier D.C., et al. Microrna-21 mediates isoflurane-induced cardioprotection against ischemia-reperfusion injury via akt/nitric oxide synthase/mitochondrial permeability transition pore pathway. Anesthesiology. 2015;123:786–798. doi: 10.1097/ALN.0000000000000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma N., Bai J., Zhang W., Luo H., Zhang X., Liu D., Qiao C. Trimetazidine protects against cardiac ischemia/reperfusion injury via effects on cardiac mirna21 expression, akt and the bcl2/bax pathway. Mol. Med. Rep. 2016;14:4216–4222. doi: 10.3892/mmr.2016.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seo H.H., Lee S.Y., Lee C.Y., Kim R., Kim P., Oh S., Lee H., Lee M.Y., Kim J., Kim L.K., et al. Exogenous mirna-146a enhances the therapeutic efficacy of human mesenchymal stem cells by increasing vascular endothelial growth factor secretion in the ischemia/reperfusion-injured heart. J. Vasc. Res. 2017;54:100–108. doi: 10.1159/000461596. [DOI] [PubMed] [Google Scholar]
- 97.Chu B., Zhou Y., Zhai H., Li L., Sun L., Li Y. The role of microrna-146a in regulating the expression of irak1 in cerebral ischemia-reperfusion injury. Can. J. Physiol. Pharmacol. 2018;96:611–617. doi: 10.1139/cjpp-2017-0586. [DOI] [PubMed] [Google Scholar]
- 98.Perry M.M., Williams A.E., Tsitsiou E., Larner-Svensson H.M., Lindsay M.A. Divergent intracellular pathways regulate interleukin-1beta-induced mir-146a and mir-146b expression and chemokine release in human alveolar epithelial cells. FEBS Lett. 2009;583:3349–3355. doi: 10.1016/j.febslet.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 99.Yang L., Wang B., Zhou Q., Wang Y., Liu X., Liu Z., Zhan Z. Microrna-21 prevents excessive inflammation and cardiac dysfunction after myocardial infarction through targeting kbtbd7. Cell Death Dis. 2018;9:769. doi: 10.1038/s41419-018-0805-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng Y., Liu X., Zhang S., Lin Y., Yang J., Zhang C. Microrna-21 protects against the H2O2-induced injury on cardiac myocytes via its target gene pdcd4. J. Mol. Cell. Cardiol. 2009;47:5–14. doi: 10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brudecki L., Ferguson D.A., McCall C.E., El Gazzar M. Microrna-146a and rbm4 form a negative feed-forward loop that disrupts cytokine mrna translation following tlr4 responses in human thp-1 monocytes. Immunol. Cell. Biol. 2013;91:532–540. doi: 10.1038/icb.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang W., Shao M., He X., Wang B., Li Y., Guo X. Overexpression of microrna-146 protects against oxygen-glucose deprivation/recovery-induced cardiomyocyte apoptosis by inhibiting the nf-kappab/tnf-alpha signaling pathway. Mol. Med. Rep. 2018;17:1913–1918. doi: 10.3892/mmr.2017.8073. [DOI] [PubMed] [Google Scholar]
- 103.Rong Y., Bao W., Shan Z., Liu J., Yu X., Xia S., Gao H., Wang X., Yao P., Hu F.B., et al. Increased microrna-146a levels in plasma of patients with newly diagnosed type 2 diabetes mellitus. PLoS ONE. 2013;8:e73272. doi: 10.1371/journal.pone.0073272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sekar D., Venugopal B., Sekar P., Ramalingam K. Role of microrna 21 in diabetes and associated/related diseases. Gene. 2016;582:14–18. doi: 10.1016/j.gene.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 105.Chien H.Y., Lee T.P., Chen C.Y., Chiu Y.H., Lin Y.C., Lee L.S., Li W.C. Circulating microrna as a diagnostic marker in populations with type 2 diabetes mellitus and diabetic complications. J. Chin. Med. Assoc. 2015;78:204–211. doi: 10.1016/j.jcma.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 106.Tu Y., Wan L., Fan Y., Wang K., Bu L., Huang T., Cheng Z., Shen B. Ischemic postconditioning-mediated mirna-21 protects against cardiac ischemia/reperfusion injury via pten/akt pathway. PLoS ONE. 2013;8:e75872. doi: 10.1371/journal.pone.0075872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toldo S., Das A., Mezzaroma E., Chau V.Q., Marchetti C., Durrant D., Samidurai A., Van Tassell B.W., Yin C., Ockaili R.A., et al. Induction of microrna-21 with exogenous hydrogen sulfide attenuates myocardial ischemic and inflammatory injury in mice. Circ. Cardiovasc. Genet. 2014;7:311–320. doi: 10.1161/CIRCGENETICS.113.000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Q., Qiu F., Zhou K., Matlock H.G., Takahashi Y., Rajala R.V.S., Yang Y., Moran E., Ma J.X. Pathogenic role of microrna-21 in diabetic retinopathy through downregulation of pparalpha. Diabetes. 2017;66:1671–1682. doi: 10.2337/db16-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feng B., Chen S., McArthur K., Wu Y., Sen S., Ding Q., Feldman R.D., Chakrabarti S. Mir-146a-mediated extracellular matrix protein production in chronic diabetes complications. Diabetes. 2011;60:2975–2984. doi: 10.2337/db11-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xie Y., Chu A., Feng Y., Chen L., Shao Y., Luo Q., Deng X., Wu M., Shi X., Chen Y. Microrna-146a: A comprehensive indicator of inflammation and oxidative stress status induced in the brain of chronic t2dm rats. Front. Pharmacol. 2018;9:478. doi: 10.3389/fphar.2018.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Palomer X., Capdevila-Busquets E., Botteri G., Davidson M.M., Rodriguez C., Martinez-Gonzalez J., Vidal F., Barroso E., Chan T.O., Feldman A.M., et al. Mir-146a targets fos expression in human cardiac cells. Dis. Model. Mech. 2015;8:1081–1091. doi: 10.1242/dmm.020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feng B., Chen S., Gordon A.D., Chakrabarti S. Mir-146a mediates inflammatory changes and fibrosis in the heart in diabetes. J. Mol. Cell. Cardiol. 2017;105:70–76. doi: 10.1016/j.yjmcc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 113.Bhaumik D., Scott G.K., Schokrpur S., Patil C.K., Orjalo A.V., Rodier F., Lithgow G.J., Campisi J. Micrornas mir-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany NY) 2009;1:402–411. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Olivieri F., Lazzarini R., Recchioni R., Marcheselli F., Rippo M.R., Di Nuzzo S., Albertini M.C., Graciotti L., Babini L., Mariotti S. Mir-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age. 2013;35:1157–1172. doi: 10.1007/s11357-012-9440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chassin C., Hempel C., Stockinger S., Dupont A., Kubler J.F., Wedemeyer J., Vandewalle A., Hornef M.W. Microrna-146a-mediated downregulation of irak1 protects mouse and human small intestine against ischemia/reperfusion injury. EMBO Mol. Med. 2012;4:1308–1319. doi: 10.1002/emmm.201201298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang Z., Wu S., Kong F., Cai X., Ye B., Shan P., Huang W. Microrna-21 protects against cardiac hypoxia/reoxygenation injury by inhibiting excessive autophagy in h9c2 cells via the akt/mtor pathway. J. Cell. Mol. Med. 2017;21:467–474. doi: 10.1111/jcmm.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu M., Li Z., Liang B., Li L., Liu S., Tan W., Long J., Tang F., Chu C., Yang J. Hydrogen sulfide ameliorates rat myocardial fibrosis induced by thyroxine through pi3k/akt signaling pathway. Endocr. J. 2018:EJ17-0445. doi: 10.1507/endocrj.EJ17-0445. [DOI] [PubMed] [Google Scholar]
- 118.Lorenzen J.M., Schauerte C., Hubner A., Kolling M., Martino F., Scherf K., Batkai S., Zimmer K., Foinquinos A., Kaucsar T., et al. Osteopontin is indispensible for ap1-mediated angiotensin ii-related mir-21 transcription during cardiac fibrosis. Eur. Heart J. 2015;36:2184–2196. doi: 10.1093/eurheartj/ehv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cao W., Shi P., Ge J.J. Mir-21 enhances cardiac fibrotic remodeling and fibroblast proliferation via cadm1/stat3 pathway. BMC Cardiovasc. Disord. 2017;17:88. doi: 10.1186/s12872-017-0520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M., Galuppo P., Just S., Rottbauer W., Frantz S., et al. Microrna-21 contributes to myocardial disease by stimulating map kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 121.Xu X., Kriegel A.J., Jiao X., Liu H., Bai X., Olson J., Liang M., Ding X. Mir-21 in ischemia/reperfusion injury: A double-edged sword? Physiol. Genomics. 2014;46:789–797. doi: 10.1152/physiolgenomics.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shu L., Zhang W., Huang G., Huang C., Zhu X., Su G., Xu J. Troxerutin attenuates myocardial cell apoptosis following myocardial ischemia-reperfusion injury through inhibition of mir-146a-5p expression. J. Cell. Physiol. 2018 doi: 10.1002/jcp.27607. [DOI] [PubMed] [Google Scholar]
- 123.D’Agostino M., Martino F., Sileno S., Barilla F., Beji S., Marchetti L., Gangi F.M., Persico L., Picozza M., Montali A., et al. Circulating mir-200c is up-regulated in paediatric patients with familial hypercholesterolaemia and correlates with mir-33a/b levels: Implication of a zeb1-dependent mechanism. Clin. Sci. (Lond) 2017;131:2397–2408. doi: 10.1042/CS20171121. [DOI] [PubMed] [Google Scholar]
- 124.Belgardt B.F., Ahmed K., Spranger M., Latreille M., Denzler R., Kondratiuk N., von Meyenn F., Villena F.N., Herrmanns K., Bosco D., et al. The microrna-200 family regulates pancreatic beta cell survival in type 2 diabetes. Nat. Med. 2015;21:619–627. doi: 10.1038/nm.3862. [DOI] [PubMed] [Google Scholar]
- 125.Magenta A., Ciarapica R., Capogrossi M.C. The emerging role of mir-200 family in cardiovascular diseases. Circ. Res. 2017;120:1399–1402. doi: 10.1161/CIRCRESAHA.116.310274. [DOI] [PubMed] [Google Scholar]
- 126.Magenta A., Cencioni C., Fasanaro P., Zaccagnini G., Greco S., Sarra-Ferraris G., Antonini A., Martelli F., Capogrossi M.C. Mir-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via zeb1 inhibition. Cell Death Differ. 2011;18:1628–1639. doi: 10.1038/cdd.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carlomosti F., D’Agostino M., Beji S., Torcinaro A., Rizzi R., Zaccagnini G., Maimone B., Di Stefano V., De Santa F., Cordisco S., et al. Oxidative stress-induced mir-200c disrupts the regulatory loop among sirt1, foxo1, and enos. Antioxid. Redox Signal. 2017;27:328–344. doi: 10.1089/ars.2016.6643. [DOI] [PubMed] [Google Scholar]
- 128.Reddy M.A., Jin W., Villeneuve L., Wang M., Lanting L., Todorov I., Kato M., Natarajan R. Pro-inflammatory role of microrna-200 in vascular smooth muscle cells from diabetic mice. Arterioscler. Thromb. Vasc. Biol. 2012;32:721–729. doi: 10.1161/ATVBAHA.111.241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang H., Liu J., Qu D., Wang L., Luo J.Y., Lau C.W., Liu P., Gao Z., Tipoe G.L., Lee H.K., et al. Inhibition of mir-200c restores endothelial function in diabetic mice through suppression of cox-2. Diabetes. 2016;65:1196–1207. doi: 10.2337/db15-1067. [DOI] [PubMed] [Google Scholar]
- 130.Lee S.T., Chu K., Jung K.H., Yoon H.J., Jeon D., Kang K.M., Park K.H., Bae E.K., Kim M., Lee S.K., et al. Micrornas induced during ischemic preconditioning. Stroke. 2010;41:1646–1651. doi: 10.1161/STROKEAHA.110.579649. [DOI] [PubMed] [Google Scholar]
- 131.Feng B., Cao Y., Chen S., Chu X., Chu Y., Chakrabarti S. Mir-200b mediates endothelial-to-mesenchymal transition in diabetic cardiomyopathy. Diabetes. 2016;65:768–779. doi: 10.2337/db15-1033. [DOI] [PubMed] [Google Scholar]
- 132.Dantas da Costa E.S.M.E., Polina E.R., Crispim D., Sbruzzi R.C., Lavinsky D., Mallmann F., Martinelli N.C., Canani L.H., Dos Santos K.G. Plasma levels of mir-29b and mir-200b in type 2 diabetic retinopathy. J. Cell. Mol. Med. 2019;23:1280–1287. doi: 10.1111/jcmm.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang J., Huang W., Xu R., Nie Y., Cao X., Meng J., Xu X., Hu S., Zheng Z. Microrna-24 regulates cardiac fibrosis after myocardial infarction. J. Cell. Mol. Med. 2012;16:2150–2160. doi: 10.1111/j.1582-4934.2012.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Qian L., Van Laake L.W., Huang Y., Liu S., Wendland M.F., Srivastava D. Mir-24 inhibits apoptosis and represses bim in mouse cardiomyocytes. J. Exp. Med. 2011;208:549–560. doi: 10.1084/jem.20101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu H., Fan G.C. Role of micrornas in the reperfused myocardium towards post-infarct remodelling. Cardiovasc. Res. 2012;94:284–292. doi: 10.1093/cvr/cvr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shantikumar S., Caporali A., Emanueli C. Role of micrornas in diabetes and its cardiovascular complications. Cardiovasc. Res. 2012;93:583–593. doi: 10.1093/cvr/cvr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jansen F., Wang H., Przybilla D., Franklin B.S., Dolf A., Pfeifer P., Schmitz T., Flender A., Endl E., Nickenig G., et al. Vascular endothelial microparticles-incorporated micrornas are altered in patients with diabetes mellitus. Cardiovasc. Diabetol. 2016;15:49. doi: 10.1186/s12933-016-0367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Babu S.S., Thandavarayan R.A., Joladarashi D., Jeyabal P., Krishnamurthy S., Bhimaraj A., Youker K.A., Krishnamurthy P. Microrna-126 overexpression rescues diabetes-induced impairment in efferocytosis of apoptotic cardiomyocytes. Sci. Rep. 2016;6:36207. doi: 10.1038/srep36207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shi J., Bei Y., Kong X., Liu X., Lei Z., Xu T., Wang H., Xuan Q., Chen P., Xu J., et al. Mir-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics. 2017;7:664–676. doi: 10.7150/thno.15162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Song T., Chen M., Rao Z., Qiu Y., Liu J., Jiang Y., Huang Z., Wang X., Lin T. Mir-17-92 ameliorates renal ischemia reperfusion injury. Kaohsiung J. Med. Sci. 2018;34:263–273. doi: 10.1016/j.kjms.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 141.Hao J., Wei Q., Mei S., Li L., Su Y., Mei C., Dong Z. Induction of microrna-17-5p by p53 protects against renal ischemia-reperfusion injury by targeting death receptor 6. Kidney Int. 2017;91:106–118. doi: 10.1016/j.kint.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li S., Zhang J., Wang Z., Wang T., Yu Y., He J., Zhang H., Yang T., Shen Z. Microrna-17 regulates autophagy to promote hepatic ischemia/reperfusion injury via suppression of signal transductions and activation of transcription-3 expression. Liver Transplant. 2016;22:1697–1709. doi: 10.1002/lt.24606. [DOI] [PubMed] [Google Scholar]
- 143.Zhu H.J., Wang D.G., Yan J., Xu J. Up-regulation of microrna-135a protects against myocardial ischemia/reperfusion injury by decreasing txnip expression in diabetic mice. Am. J. Translant. Res. 2015;7:2661–2671. [PMC free article] [PubMed] [Google Scholar]
- 144.Cheng Y., Sun T., Yin C., Wang S., Li Z., Tao Y., Zhang J., Li Z., Zhang H. Downregulation of pten by sodium orthovanadate protects the myocardium against ischemia/reperfusion injury after chronic atorvastatin treatment. J. Cell. Biochem. 2019;120:3709–3715. doi: 10.1002/jcb.27651. [DOI] [PubMed] [Google Scholar]
- 145.Hao Y.L., Fang H.C., Zhao H.L., Li X.L., Luo Y., Wu B.Q., Fu M.J., Liu W., Liang J.J., Chen X.H. The role of microrna-1 targeting of mapk3 in myocardial ischemia-reperfusion injury in rats undergoing sevoflurane preconditioning via the pi3k/akt pathway. Am. J. Physiol. Cell. Physiol. 2018;315:C380–C388. doi: 10.1152/ajpcell.00310.2017. [DOI] [PubMed] [Google Scholar]
- 146.Zaccagnini G., Maimone B., Fuschi P., Maselli D., Spinetti G., Gaetano C., Martelli F. Overexpression of mir-210 and its significance in ischemic tissue damage. Sci. Rep. 2017;7:9563. doi: 10.1038/s41598-017-09763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Di Y.F., Li D.C., Shen Y.Q., Wang C.L., Zhang D.Y., Shang A.Q., Hu T. Mir-146b protects cardiomyocytes injury in myocardial ischemia/reperfusion by targeting smad4. Am. J. Translant. Res. 2017;9:656–663. [PMC free article] [PubMed] [Google Scholar]
- 148.Zuo Y., Wang Y., Hu H., Cui W. Atorvastatin protects myocardium against ischemia-reperfusion injury through inhibiting mir-199a-5p. Cell. Physiol. Biochem. 2016;39:1021–1030. doi: 10.1159/000447809. [DOI] [PubMed] [Google Scholar]
- 149.Park K.M., Teoh J.P., Wang Y., Broskova Z., Bayoumi A.S., Tang Y., Su H., Weintraub N.L., Kim I.M. Carvedilol-responsive micrornas, mir-199a-3p and -214 protect cardiomyocytes from simulated ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2016;311:H371–H383. doi: 10.1152/ajpheart.00807.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zeng X.C., Li L., Wen H., Bi Q. Microrna-128 inhibition attenuates myocardial ischemia/reperfusion injury-induced cardiomyocyte apoptosis by the targeted activation of peroxisome proliferator-activated receptor gamma. Mol. Med. Rep. 2016;14:129–136. doi: 10.3892/mmr.2016.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pan Z., Sun X., Ren J., Li X., Gao X., Lu C., Zhang Y., Sun H., Wang Y., Wang H., et al. Mir-1 exacerbates cardiac ischemia-reperfusion injury in mouse models. PLoS ONE. 2012;7:e50515. doi: 10.1371/journal.pone.0050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ye Y., Hu Z., Lin Y., Zhang C., Perez-Polo J.R. Downregulation of microrna-29 by antisense inhibitors and a ppar-gamma agonist protects against myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2010;87:535–544. doi: 10.1093/cvr/cvq053. [DOI] [PubMed] [Google Scholar]
- 153.Hu S., Huang M., Li Z., Jia F., Ghosh Z., Lijkwan M.A., Fasanaro P., Sun N., Wang X., Martelli F. Microrna-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122:S124–S131. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang X., Zhang X., Ren X.-P., Chen J., Liu H., Yang J., Medvedovic M., Hu Z., Fan G.-C. Microrna-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injuryclinical perspective. Circulation. 2010;122:1308–1318. doi: 10.1161/CIRCULATIONAHA.110.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ren X.-P., Wu J., Wang X., Sartor M.A., Qian J., Jones K., Nicolaou P., Pritchard T.J., Fan G.-C. Microrna-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang L., Niu X., Hu J., Xing H., Sun M., Wang J., Jian Q., Yang H. After myocardial ischemia-reperfusion, mir-29a, and let7 could affect apoptosis through regulating igf-1. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/245412. [DOI] [PMC free article] [PubMed] [Google Scholar]