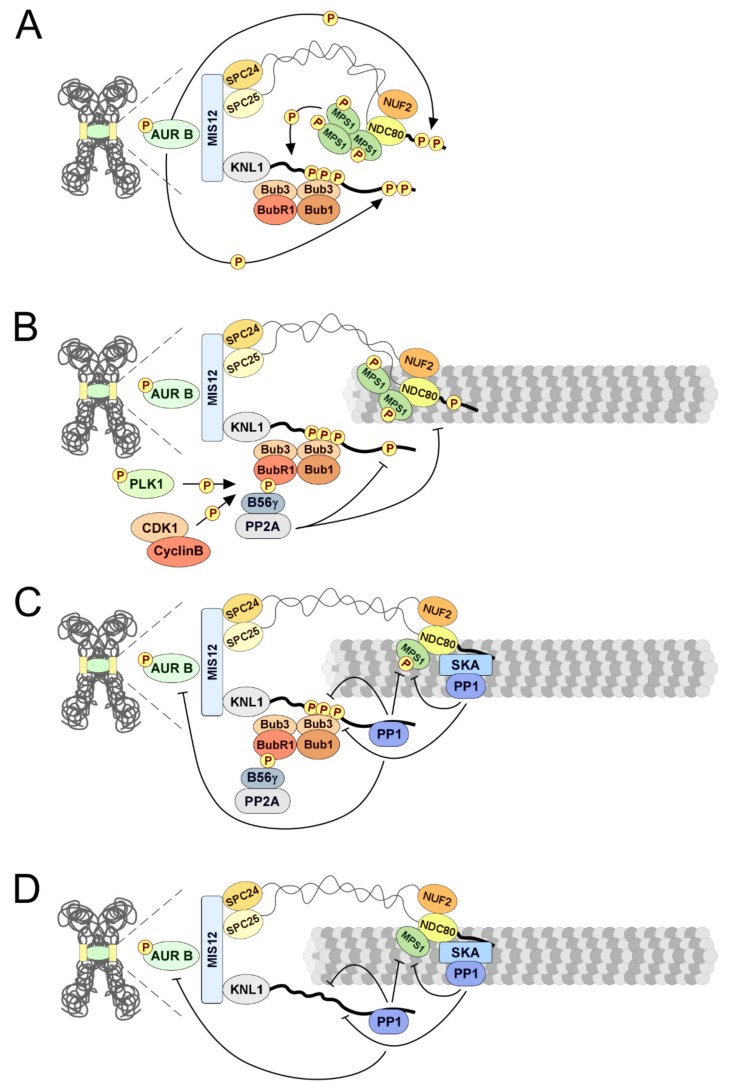
Figure 6.
Kinetochore PP2A-B56 and PP1 promote the stability of microtubule attachments and efficient Spindle Assembly Checkpoint silencing in animal cells. (A,B) In the absence of microtubules, MPS1 kinase efficiently accumulates at kinetochores through direct binding to the NDC80 complex. This enables MPS1 trans-autoactivation and instates SAC signaling. MPS1 phosphorylates several MELT motifs on KNL1, generating docking sites for the hierarchical recruitment of SAC proteins required for MCC assembly. This also enables the recruitment of PP2A-B56γ to kinetochores, following PLK1-mediated phosphorylation of BUBR1 KARD motif. PP2A-B56γ bound to BUBR1 has critical roles in allowing the initial binding of microtubules to NDC80 and in establishing SAC responsiveness. The activity of PP2A-B56γ-BUBR1 antagonizes microtubule-destabilizing phosphorylations on the NDC80 N-terminal tail. These phosphorylations are catalyzed by centromeric Aurora B and predominate at tensionless kinetochores to enable the correction of erroneous attachments. Dephosphorylation of the NDC80 N-terminal tail by PP2A-B56-BUBR1 provides NDC80 an opportunity to bind microtubules. (C,D) As microtubules occupancy increases, kinetochore tension or alterations on the kinetochore architecture place outer-kinetochore proteins further apart from the Aurora B zone of influence. This renders PP2A-B56γ-BUBR1 activity largely unopposed, which ultimately results in complete dephosphorylation of NDC80 and in the formation of stable end-on attachments. At this point, several pools of PP1γ accumulate at the kinetochore to preserve the stability of bioriented attachments and promote prompt SAC silencing. The recruitment of PP1γ is mediated in part by its interaction with PP1-binding motifs on the KNL1 N-terminus. PP1γ-KNL1 dephosphorylates phospho-MELTs, causing the dissociation of SAC proteins from kinetochores and consequently ceasing MCC assembly. Binding of PP1γ to KNL1 is limited at unattached or tensionless kinetochores due to Aurora B-mediated phosphorylation of both PP1-docking motifs, which significantly represses their ability to interact with the phosphatase. These phosphorylations are counteracted by the activity of PP2A-B56γ bound to BUBR1. Thus, as end-on attachments are established, the KNL1 N-terminus becomes inaccessible to Aurora B and PP2A-B56γ ensures efficient recruitment of PP1γ. Increasing levels of PP1γ-KNL1 further suppress Aurora B activity at kinetochores, hence enhancing the stability of microtubule attachments. PP1γ-KNL1 is also proposed to directly antagonize the activating T-loop autophosphorylation of Aurora B, which likely favors the recruitment of the SKA complex to dephosphorylated NDC80. The SKA complex directly binds PP1γ through its SKA1 subunit and independently of KNL1, thus representing an additional pathway to deliver PP1γ to kinetochores of bioriented chromosomes. Importantly, because MPS1 and microtubules have partially overlapping binding surfaces on the NDC80 complex, the formation of stable end-on attachments imposes a substantial reduction in the amount of MPS1 at kinetochores. Residual MPS1 molecules remaining on bioriented kinetochores are dephosphorylated and inactivated by PP1. These mechanisms concur to efficiently avert SAC function at the top of the signaling cascade.