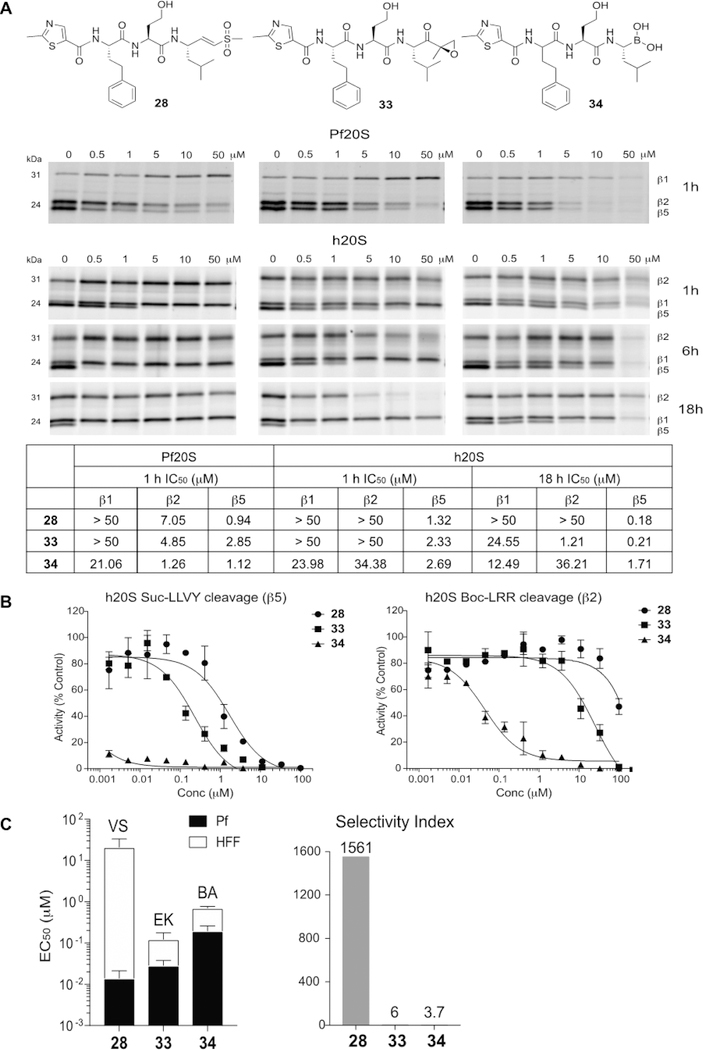
Figure 4.
Choice of electrophile is important for obtaining optimal selectivity of proteasome inhibitors. (A) Structures of compounds 28, 33, and 34 and inhibition of purified P. falciparum and human proteasome 20S as assessed by competitive activity-based probe labeling. Purified 20S proteasomes were incubated with each inhibitor for indicated times followed by labeling of residual proteasome activity using the activity-based probes. The IC50 values were calculated for each inhibitor for the P. falciparum and human 20S proteasomes by quantifying labeled proteins using ImageJ and normalizing them to mock-treated control. (B) Inhibition of compounds 28, 33, and 34 for processing of the fluorogenic substrates, Suc-LLVY-AMC (β5) and Boc-LRR-AMC (β2) by human proteasome 20S. The inhibitors were incubated with h20S for 1 h followed by the addition of each fluorogenic substrate. The AMC molecules released by residual proteasomal activity were measured (excitation at 380 nm, emission at 460 nm) and the slope over the linear range of the reaction was used for the calculation of IC50 values using Prism. (C) A stacked bar plot of EC50 values (logarithmic scale) for the vinyl sulfone (VS)-, epoxyketone (EK)- and boronic acid (BA) derivatives 28, 33, and 34 in 72 h treatment of P. falciparum W2 at ring stage parasites (black bar) and nonconfluent HFFs (white bar) with error bars, s.d. (left) and selectivity index (comparing the P. falciparum activity to HFF activity, HFF EC50/P. falciparum EC50, right).