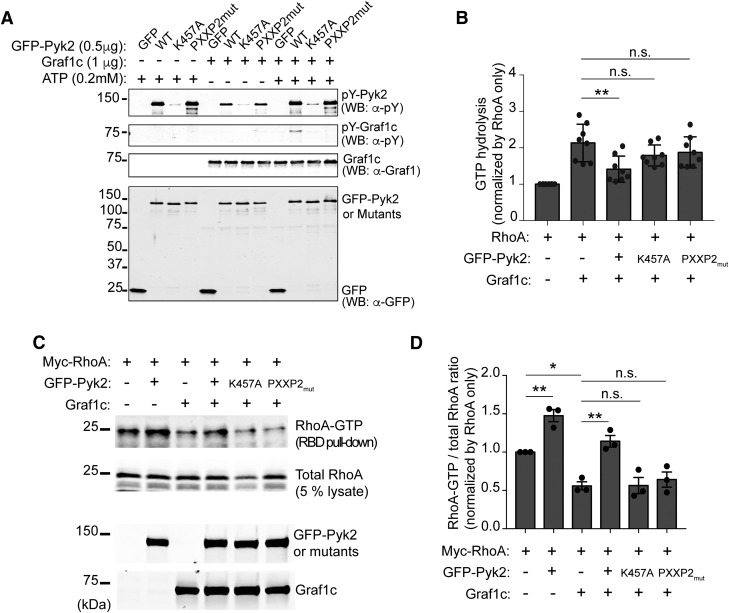
Figure 6.
Pyk2 induces RhoA activation through Graf1c interaction and phosphorylation. A, In vitro Pyk2 kinase assay with recombinant Graf1c as a substrate. Purified Graf1c from bacteria and GFP or GFP tagged Pyk2, K457A, and PXXP2mut from Hek293T cells were incubated in the absence or presence of ATP for 30 min at room temperature. Proteins were separated by SDS-PAGE and immunoblotted with indicated antibodies. B, In vitro RhoGAP assay with recombinant RhoA protein. The phosphate generated by the hydrolysis of GTP by RhoA GTPase is measured to determine the Graf1c GAP activity. The y-axis represents the relative ratio in indicated reactions compared with in RhoA only. Data are graphed as mean ± SEM (n = 8 experiments). **p < 0.01 by one-way ANOVA, Tukey's multiple-comparisons test. C, GST-RBD pull-down assay from Hek293T cell lysate from cells transfected with the indicated expression plasmids. The proteins retained by GST-RBD-immobilized beads and lysates were subjected to immunoblotting with anti-Myc, anti-Pyk2, anti-Graf1 antibodies. D, Levels of RhoA-GTP (RBD pull-down) and RhoA-total (5% lysate) were quantified by densitometric measurement. The y-axis represents the relative ratio in indicated cells compared with in Myc-RhoA only expressing cells. Data are graphed as mean ± SEM (n = 3 experiments). *p < 0.05, **p < 0.01 by one-way ANOVA, Tukey's multiple-comparisons test.