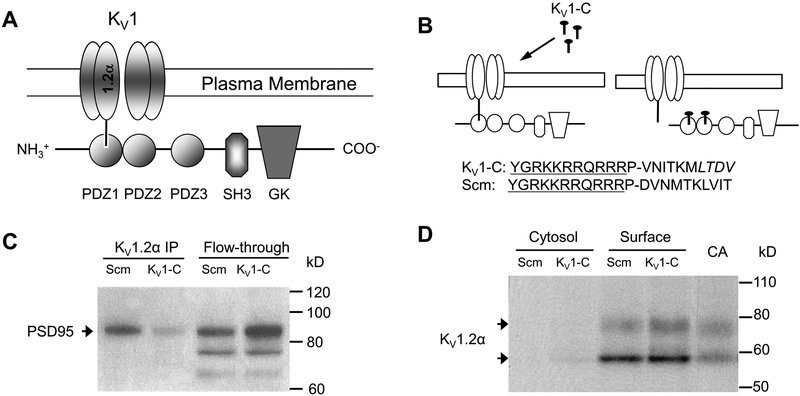
Figure 1. PSD95 interaction with the Kv1 channel and disruption by Kv1-C peptide.
A) Schematic of the association of the Kv1 channel α1.2 subunit with the PSD95 scaffold via the PDZ1 binding domain. PSD95 contains three PDZ binding domains (PDZ1–3), and Src-homology (SH3) and guanylate kinase (GK) domains. B) The Kv1-C dominant negative peptide was designed to compete for the PDZ binding domain on PSD95. The last 10 amino acids of the C-terminus of the α1.2 pore protein were conjugated to HIV-tat (YGRKKRRQRRR) to confer cell-permeability. P is a spacer. LTDV is a class-1 PDZ binding motif on the α1.2 subunit. A peptide with same amino acid composition in a scrambled order (Scm) was used as control. C) Immunoprecipitation using anti-Kvα1.2 of rat cerebral arterial lysate treated with Scm or Kv1-C peptide for 30 min. The Kvα1.2 immunoprecipitate and column flow-through (Flow-through) were probed for PSD95 on a Western blot. Depicted is a representative scan from three similar experiments showing that Kv1-C peptide disrupted PSD95 association with α1.2. D) Biotinylation of rat cerebral arteries treated with Scm or Kv1-C peptide for 30 min. Cytosolic and surface fractions were probed for the Kv1 channel α1.2 subunit. Control lysate from freshly isolated cerebral arteries (CA) was loaded for size comparison. Depicted is a representative blot from five similar experiments. Kv1-C did not alter the surface expression of Kv1 channel α1.2 subunits, which appear as a doublet band at ~ 58 kD and 80 kD; the upper band represents the glycosylated form. Figures and legend from reference 82.