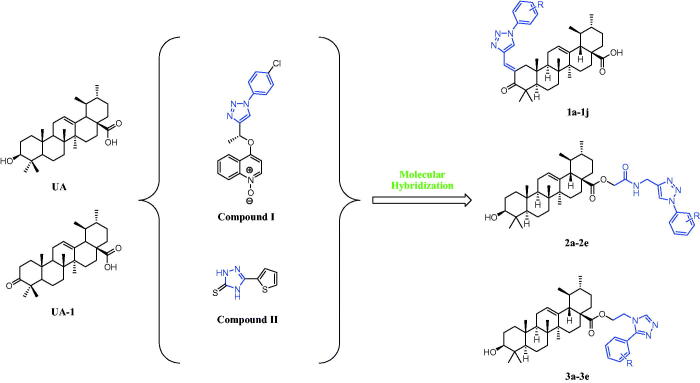
Abstract
Ursolic acid (UA), a plant-derived compound, has many properties beneficial to health. In the present study, we synthesised three series of novel UA derivatives and evaluated their anti-Toxoplasma gondii activity both in vitro and in vivo. Most derivatives exhibited an improved anti-T. gondii activity in vitro when compared with UA (parent compound), whereas compound 3d exhibited the most potent anti-T. gondii activity in vivo. Spiramycin served as the positive control. Additionally, determination of biochemical parameters, including the liver and spleen indexes, indicated compound 3d to effectively reduce hepatotoxicity and significantly enhance anti-oxidative effects, as compared with UA. Furthermore, our molecular docking study indicated compound 3d to possess a strong binding affinity for T. gondii calcium-dependent protein kinase 1 (TgCDPK1). Based on these findings, we conclude that compound 3d, a derivative of UA, could act as a potential inhibitor of TgCDPK1.
Keywords: Toxoplasma gondii, ursolic acid, molecular docking, TgCDPK1, in vivo, in vitro
1. Introduction
Toxoplasma gondii is an opportunistic pathogen that causes infection in human beings and various animals, thereby severely impairing their health. Congenital toxoplasmosis, caused by T. gondii, is especially harmful to pregnant women as the infection may result in abortion, stillbirth, and abnormality of foetus thinking barrier. The infection could also be fatal for immuno-compromised patients1. Owing to the complexity of T. gondii life cycle, its multifarious pathogenesis and different biological characteristics, no preventive and medicine-specific treatment exists currently. Traditional anti-T. gondii drugs have various disadvantages, such as the inability to completely kill the protozoa and oocysts, high toxicity, frequent recurrence, and failure in immuno-compromised individuals2,3. Considering the increasing percentage of natural product-based drugs in the market in the past years, researchers have now focussed their attention to plant-based compounds with anti-T. gondii activity. Moreover, several studies have shown natural products and their derivatives to exert strong anti-T. gondii effects, making these an attractive source of anti-T. gondii drugs4,5. In this regard, structural modifications of natural products to generate effective and less-toxic derivatives are considered to be very promising for the development of anti-T. gondii drugs.
Pentacyclic triterpenes are a diverse and large class of natural products that are widely distributed in the plant kingdom. Over the decades, the synthesis of novel pentacyclic triterpenes has gained much attention in medicinal chemistry. Among these, ursolic acid (UA) and its derivatives have been reported to possess a wide range of biological activities, including anti-cancer6,7, anti-diabetic8, anti-HIV9, anti-malarial10, anti-microbial, and anti-inflammatory activities11,12. Until recently, Choi et al. reported that UA not only has strong anti-proliferative activity against T. gondii activity as well as increases survival of T. gondii-infected mice but also has the potential to be used as a promising anti-T. gondii candidate for developing effective anti-parasitic drugs13. To the best of our knowledge, studies related to anti-T. gondii activity of any UA derivatives have not yet been reported. Besides, the higher cytotoxicity in vitro and the low bioavailability in vivo of UA restrict its clinical application14,15. Therefore, the present study involved synthesis of different structurally modified compounds of UA with significantly improved anti-T. gondii activity and lower toxicity.
Recently, the chemistry of triazoles and their fused phenyl derivatives has received considerable attention owing to their effective biological and synthetic importance16–18. Sharling et al. reported that a series of 1,2,3-triazoles conjugate phenyl derivatives facilitated the development of potential anti-parasitic agents, of which, five derivatives exhibited excellent in vitro selectivity for T. gondii. Among these, compound 1 (Figure 1) exhibited the most potent anti-T. gondii activity with a selectivity value of more than 12018. Furthermore, Dzitko et al. reported anti-T. gondii activity of 3-(thiophen-2-yl)-1,2,4-triazole-5-thione (compound 2). The compound displayed significant and reproducible anti-parasitic effects in vitro, with selectivity values of 4.58 and 5.21 using 3[H]uracil incorporation method and qRT-PCR, respectively19. These studies indicate triazole-based compounds to have potential inhibitory activity against anti-T. gondii.
Figure 1.
Design of target compounds based on the combination principles.
The aforementioned findings stimulated our interest in designing and synthesising three series of novel UA derivatives by linking different fragments containing 1,2,3-triazole and 1,2,4-triazole and studying their effects against T. gondii, initially at the cellular level. We next tested each of these derivatives for the strongest anti-T. gondii activity in vivo, since in vivo effects are an important factor in evaluating anti-parasitic activity. Finally, we aimed to gain a better understanding of the molecular basis of inhibitory potency of compounds against T. gondii. For this, we identified three enzymes through literature search as reasonable targets for discovering anti-T. gondii agents, and by using the molecular docking approach, we aimed at finding the possible target.
2. Materials and methods
2.1. General procedures
All reactions were monitored by thin-layer chromatography (TLC) performed on silica gel plates. Melting points were determined in open capillary tubes and were uncorrected. Purity of final products was determined using a preparative high-performance liquid chromatography (HPLC) system (HP-Q-P050; Agela Technologies) with a C-18 column as the stationary phase (Agela Technologies, Venusil PrepG, 120 Å, 10 μm, 10 mm × 250 mm). The nuclear magnetic resonance (1H-NMR and 13C-NMR) spectra were recorded with AV-300 spectrometers (Bruker BioSpin, Switzerland); all chemical shifts were expressed in ppm relative to tetramethylsilane (TMS), used as the internal standard. High-resolution mass spectra were recorded using the Thermo Scientific LTQ Orbitrap XL in the electrospray ionisation (ESI) mode. Major chemicals were purchased from Aldrich Chemical Corporation (Milwaukee, WI, USA). All other chemicals were of analytical grade.
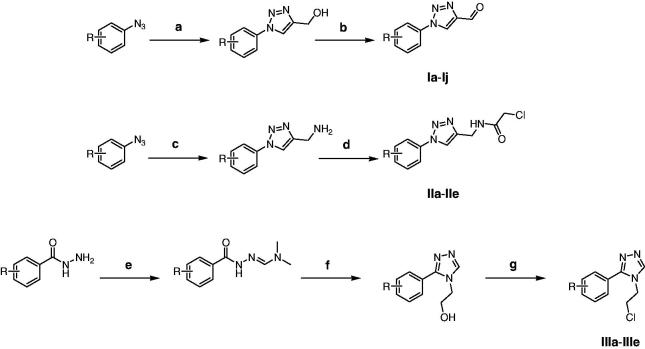
2.2. General procedure for synthesis of intermediates (UA-1, Ia-Ij, IIa-IIe, IIIa-IIIe)
The compound UA-1 was synthesised as per the protocol described in a previous study 6. Ia-Ij (different 1-phenyl-1H-1,2,3-triazole-4-carbaldehyde) and IIa-IIe (different 2-chloro-N-((1-phenyl-1H-1,2,3-triazol-4-yl)methyl)acetamide) were prepared as previously described17,20. IIIa-IIIe were prepared as per Scheme 1: different substitutions of benzoyl hydrazide (10 mmol) and N,N-Dimethylformamide dimethyl acetal (DMFDMA; 1.31 g, 11 mmol) were added to CH3CN (20 ml); the resulting mixture was stirred at 60 °C for 1 h. Then, 2-aminoethanol (1.22 g, 20 mmol) and CH3COOH (2.40 g, 40 mmol) were added, and the resulting mixture was stirred at 90 °C for 8–12 h. After confirming the reaction progress by TLC, the solvent was evaporated in vacuo. The mixture was then purified using silica gel column chromatography and eluted using a gradient of dichloromethane:methanol (100:1–40:1) to obtain different 2–(3-phenyl-4H-1,2,4-triazol-4-yl)ethanol derivatives. These products were placed in CHCl3 (20 ml) and 5 molar ratios of sulfoxide chloride was added. The mixture was stirred at 60 °C for 3 h. Upon completion, the solvent and excessive sulfoxide chloride was evaporated in vacuo to obtain different intermediates, which were used in the next step without further purification.
Scheme 1.
Reagents and conditions: (a) propargyl alcohol, CuSO4.5H2O, sodium ascorbate, t-BuOH/H2O (1:1), 30 °C. (b) MnO2, EtOAc, 70 °C. (c) propynylamine, CuSO4 5H2O, sodium ascorbate, t-BuOH/H2O (1:1), 30 °C. (d) chloroacetyl chloride, Et3N, CH2Cl2, 30 °C. (e) DMFDMA, CH3CN, 60 °C. (f) 2-aminoethanol, CH3COOH, 90 °C. (g) sulfoxide chloride, CHCl3, 60 °C.
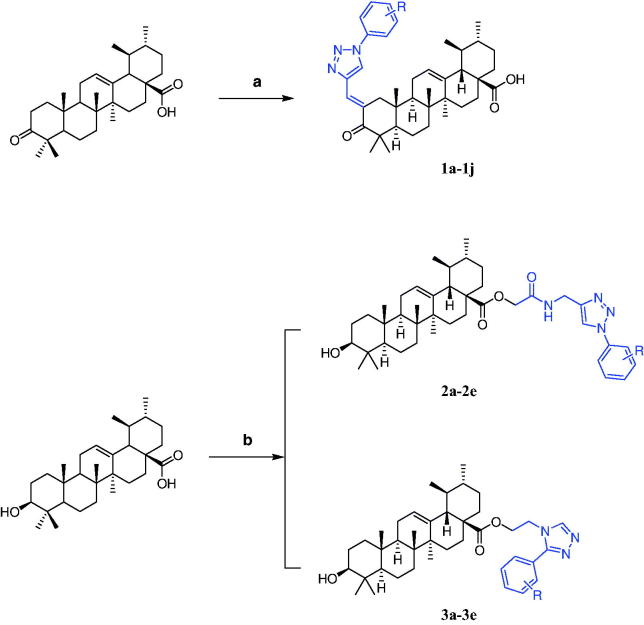
2.3. General procedure for synthesis of compound (1a–1j)
A mixture of UA-1 (90.8 mg, 0.20 mmol), KOH (112 mg, 2.0 mmol) and different 1-phenyl-1H-1,2,3-triazole-4-carbaldehydes (0.21 mmol) was prepared in CH3CH2OH (10 ml) and stirred at 30 °C for 3–5 h. Progress of reaction was confirmed by TLC, following which the solvent was evaporated in vacuo. The mixture was neutralised with hydrochloric acid, extracted with 15 ml ethyl acetate, and then washed thrice with saline (5 ml). The final products were purified using preparative HPLC equipped with a C-18 column. A gradient elution was performed with tetrahydrofuran and water as the mobile phase and was monitored at 220 nm and 254 nm. 1H and 13C-NMR spectra of all the target compounds are available in the Supplementary materials.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS,E)-1,2,6a,6b,9,9,12a-heptamethyl-10-oxo-11-((1-phenyl-1H-1,2,3-triazol-4-yl)methylene)-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylic acid (1a)
White solid; yield, 78%; m.p. >250 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.09 (s, 1H, triazole-H), 7.80 − 7.77 (m, 2H, Ar-H), 7.61 − 7.47 (m, 4H, Ar–H, –CO–C = CH–), 5.35 (s, 1H, C12–H), 3.54 (d, J = 17.4 Hz, 1H, C1–He), 2.48 (d, J = 18.0 Hz, 1H, C1–Ha), 2.27 − 2.18 (m, 2H), 2.10 − 2.00 (m, 2H), 1.94 − 1.89 (m, 1H), 1.83 − 1.69 (m, 4H), 1.58 − 1.52 (m, 4H), 1.45 − 1.27 (m, 5H), 1.19 − 1.15 (m, 9H), 0.99 − 0.92 (m, 9H), 0.89 − 0.85 (m, 4H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 207.63, 183.98, 145.17, 137.87, 136.69, 135.66, 129.89 (2C), 129.04, 125.82, 123.69, 122.94, 120.59 (2C), 53.04, 52.70, 48.11, 45.10 (2C), 44.94, 42.19, 39.38, 39.18, 38.86, 36.73, 36.04, 32.09, 30.65, 29.73, 28.03, 24.11, 23.70, 23.46, 22.61, 21.17, 20.37, 17.15, 16.75, 15.75. ESI-HRMS (m/z): calculated for C39H52N3O3+ [M + H]+: 610.4003, found: 610.4001.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS,E)-11-((1–(2-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-1,2,6a,6b,9,9,12a-heptamethyl-10-oxo-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylic acid (1b)
White solid; yield, 82%; m.p. >250 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.20 (s, 1H, triazole-H), 8.05 − 7.99 (m, 1H, Ar–H) 7.54 − 7.33 (m, 4H, Ar–H, –CO–C = CH–), 5.35 (s, 1H, C12–H), 3.50 (d, J = 17.7 Hz, 1H, C1–He), 2.47 (d, J = 18.0 Hz, 1H, C1–Ha), 2.27 − 2.18 (m, 2H), 2.10 − 2.01 (m, 2H), 1.94 − 1.89 (m, 1H), 1.83 − 1.69 (m, 4H), 1.58 − 1.52 (m, 5H), 1.46 − 1.31 (m, 4H), 1.27 − 1.15 (m, 10H), 0.99 − 0.92 (m, 10H), 0.90 − 0.87 (m, 2H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 207.55, 183.78, 154.98, 151.66, 144.91, 137.86, 135.75, 130.36, 125.83, 125.42, 124.72, 123.68, 117.30, 117.04, 53.02, 52.71, 48.10, 45.14, 45.11, 44.99, 42.19, 39.38, 39.18, 38.85, 36.73, 36.04, 32.09, 30.65, 29.71, 28.02, 24.12, 23.65, 23.45, 22.58, 21.17, 20.36, 17.10, 16.75, 15.77. ESI-HRMS (m/z): calculated for C39H51FN3O3+ [M + H]+: 628.3909, found: 628.3907.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS,E)-11-((1–(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-1,2,6a,6b,9,9,12a-heptamethyl-10-oxo-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylic acid (1c)
White solid; yield, 80%; m.p. >250 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.08 (s, 1H, triazole-H), 7.60 − 7.50 (m, 4H, Ar–H, –CO–C = CH–), 7.23 − 7.18 (m, 1H, Ar–H), 5.34 (s, 1H, C12–H), 3.54 (d, J = 16.5 Hz, 1H, C1–He), 2.48 (d, J = 16.8 Hz, 1H, C1–Ha), 2.16 − 2.19 (m, 2H), 2.09 − 2.01 (m, 2H), 1.93 − 1.90 (m, 1H), 1.83 − 1.69 (m, 4H), 1.57 − 1.52 (m, 4H), 1.45 − 1.27 (m, 5H), 1.19 − 1.15 (m, 9H), 0.99 − 0.94 (m, 9H), 0.87 (s, 4H). 13C-NMR (CDCl3, 125 MHz, ppm): δ 207.65, 183.40, 163.15, 145.41, 137.91, 137.81, 136.14, 131.38, 125.82, 123.28, 122.76, 115.97, 115.89, 108.39, 53.07, 52.76, 48.10, 45.14, 45.09, 44.94, 42.22, 39.40, 39.17, 38.87, 36.73, 36.06, 32.09, 30.65, 29.73, 28.02, 24.14, 23.71, 23.47, 22.62, 21.16, 20.39, 17.14, 16.73, 15.77. ESI-HRMS (m/z): calculated for C39H51FN3O3+ [M + H]+: 628.3909, found: 628.3906.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS,E)-11-((1–(4-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-1,2,6a,6b,9,9,12a-heptamethyl-10-oxo-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylic acid (1d)
White solid; yield, 82%; m.p. >250 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.04 (s, 1H, triazole-H), 7.79 − 7.74 (m, 2H, Ar–H), 7.51 (s, 1H, –CO–C = CH–), 7.31 − 7.25 (m, 2H, Ar–H), 5.34 (s, 1H, C12–H), 3.53 (d, J = 16.8 Hz, 1H, C1–He), 2.47 (d, J = 18.6 Hz, 1H, C1–Ha), 2.26 − 2.18 (m, 2H), 2.10 − 2.01 (m, 2H), 1.94 − 1.89 (m, 1H), 1.83 − 1.69 (m, 4H), 1.58 − 1.50 (m, 4H), 1.46 − 1.28 (m, 5H), 1.19 − 1.15 (m, 9H), 1.05 − 0.92 (m, 9H), 0.87 (s, 4H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 207.62, 183.80, 145.28, 137.91, 135.85, 132.94, 126.92, 125.78, 123.50, 123.04, 122.65, 122.54, 117.05, 116.74, 53.05, 52.71, 48.10, 45.11, 45.08, 44.92, 42.19, 39.38, 39.17, 38.86, 36.73, 36.04, 32.08, 30.64, 29.72, 28.03, 24.11, 23.70, 23.46, 22.61, 21.16, 20.36, 17.14, 16.75, 15.75. ESI-HRMS (m/z): calculated for C39H51FN3O3+ [M + H]+: 628.3909, found: 628.3907.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS,E)-11-((1–(2-chlorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-1,2,6a,6b,9,9,12a-heptamethyl-10-oxo-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylic acid (1e)
White solid; yield, 81%; m.p. >250 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.12 (s, 1H, triazole-H), 7.70 − 7.62 (m, 2H, Ar–H), 7.56 (s, 1H, –CO–C = CH–), 7.53 − 7.49 (m, 2H, Ar–H), 5.34 (s, 1H, C12–H), 3.45 (d, J = 16.8 Hz, 1H, C1–He), 2.45 (d, J = 17.4 Hz, 1H, C1–Ha), 2.26 − 2.16 (m, 2H), 2.11 − 2.01 (m, 2H), 1.94 − 1.89 (m, 1H), 1.82 − 1.68 (m, 4H), 1.58 − 1.52 (m, 4H), 1.49 − 1.27 (m, 5H), 1.20 − 1.15 (m, 9H), 0.99 − 0.96 (m, 5H), 0.94 − 0.92 (m, 3H), 0.90 − 0.85 (m, 5H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 207.56, 183.51, 144.29, 137.91, 135.65, 134.60, 130.93 (2C), 128.52, 128.07, 127.63, 126.74, 125.79, 123.87, 53.01, 52.73, 48.10, 45.18, 45.11, 45.08, 42.21, 39.38, 39.18, 38.85, 36.71, 36.03, 32.09, 30.65, 29.72, 28.02, 24.13, 23.69, 23.45, 22.55, 21.15, 20.36, 17.09, 16.75, 15.82. ESI-HRMS (m/z): calculated for C39H51ClN3O3+ [M + H]+: 644.3613, found: 644.3609.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS,E)-11-((1–(3,4-dichlorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-1,2,6a,6b,9,9,12a-heptamethyl-10-oxo-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylic acid (1f)
White solid; yield, 79%; m.p. >250 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.06 (s, 1H, triazole-H), 7.95 (s, 1H, Ar–H), 7.67 (s, 2H, Ar–H), 7.48 (s, 1H, –CO–C = CH–), 5.34 (s, 1H, C12–H), 3.54 (d, J = 15.7 Hz, 1H, C1–He), 2.47 (d, J = 15.4 Hz, 1H, C1–Ha), 2.27 − 2.16 (m, 2H), 2.10 − 2.00 (m, 2H), 1.95 − 1.89 (m, 1H), 1.83 − 1.69 (m, 4H), 1.58 − 1.50 (m, 4H), 1.45 − 1.27 (m, 5H), 1.19–1.52 (m, 9H), 1.07 − 1.04 (m, 1H), 1.00 − 0.97 (m, 3H), 0.94 − 0.92 (m, 5H), 0.87–0.81 (m, 4H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 207.58, 183.48, 145.60, 137.93, 136.39, 135.67, 134.19, 133.24, 131.62, 125.76, 123.02, 122.59, 122.35, 119.46, 53.05, 52.73, 48.10, 45.14, 45.09, 44.94, 42.21, 39.38, 39.18, 38.85, 36.72, 36.05, 32.08, 30.64, 29.70, 28.01, 24.12, 23.69, 23.45, 22.61, 21.15, 20.35, 17.12, 16.75, 15.75. ESI-HRMS (m/z): calculated for C39H50Cl2N3O3+ [M + H]+: 678.3224, found: 678.3225.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS,E)-11-((1–(2-bromophenyl)-1H-1,2,3-triazol-4-yl)methylene)-1,2,6a,6b,9,9,12a-heptamethyl-10-oxo-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylic acid (1g)
White solid; yield, 76%; m.p. >250 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.09 (s, 1H, triazole-H), 7.82 − 7.80 (m, 1H, Ar–H) 7.64 − 7.41 (m, 4H, Ar–H, –CO–C = CH–), 5.34 (s, 1H, C12–H), 3.46 (d, J = 17.1 Hz, 1H, C1–He), 2.45 (d, J = 18.0 Hz, 1H, C1–Ha), 2.27 − 2.18 (m, 2H), 2.10 − 2.01 (m, 2H), 1.94 − 1.89 (m, 1H), 1.82 − 1.66 (m, 4H), 1.58 − 1.47 (m, 6H), 1.42 − 1.32 (m, 3H), 1.21 − 1.16 (m, 9H), 1.08 − 0.97 (m, 6H), 0.94 − 0.92 (m, 3H), 0.88 − 0.82 (m, 4H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 207.62, 183.67, 145.42, 137.90, 137.52, 136.15, 135.76, 130.97, 129.10, 125.79, 123.26, 122.74, 120.86, 118.52, 53.05, 52.72, 48.10, 45.13, 45.09, 44.94, 42.10, 39.39, 39.18, 38.85, 36.73, 36.05, 32.08, 30.64, 29.71, 28.02, 24.12, 23.70, 23.46, 22.61, 21.15, 20.36, 17.12, 16.75, 15.75. ESI-HRMS (m/z): calculated for C39H51BrN3O3+ [M + H]+: 688.3108, found: 688.3106.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS,E)-11-((1–(2-iodophenyl)-1H-1,2,3-triazol-4-yl)methylene)-1,2,6a,6b,9,9,12a-heptamethyl-10-oxo-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylic acid (1h)
White solid; yield, 75%; m.p. >250 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.07 − 8.01 (m, 2H, Ar-H, triazole-H), 7.59 − 7.49 (m, 3H, Ar–H, –CO–C = CH–), 7.32 − 7.29 (m, 1H), 5.34 (s, 1H, C12–H), 3.44 (d, J = 17.7 Hz, 1H, C1–He), 2.46 (d, J = 17.7 Hz, 1H, C1–Ha), 2.27 − 2.19 (m, 2H), 2.11 − 2.00 (m, 2H), 1.95 − 1.89 (m, 1H), 1.82 − 1.68 (m, 4H), 1.58 − 1.52 (m, 4H), 1.46 − 1.27 (m, 5H), 1.20 − 1.15 (m, 9H), 1.08 − 0.97 (m, 7H), 0.93 − 0.91 (m, 3H), 0.87 (s, 3H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 207.61, 183.68, 144.22, 140.43, 139.71, 137.94, 135.66, 131.64, 129.41, 127.80, 126.64, 125.74, 124.03, 93.56, 52.96, 52.73, 48.11, 45.21, 45.12(2C), 42.21, 39.38, 39.16, 38.85, 36.71, 36.05, 32.08, 30.65, 29.73, 28.00, 24.11, 23.76, 23.46, 22.54, 21.15, 20.35, 17.14, 16.75, 15.87. ESI-HRMS (m/z): calculated for C39H51IN3O3+ [M + H]+: 736.2970, found: 736.2968.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS,E)-11-((1–(2-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methylene)-1,2,6a,6b,9,9,12a-heptamethyl-10-oxo-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylic acid (1i)
White solid; yield, 77%; m.p. >250 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.31 (s, 1H, triazole-H), 7.90 (dd, J = 7.8, 1.5 Hz, 1H, Ar–H), 7.59 (s, 1H, –CO–C = CH–), 7.47 (td, J = 8.4, 1.5 Hz, 1H, Ar–H), 7.19 − 7.12 (m, 2H, Ar–H), 5.35 (s, 1H, C12–H), 3.93 (s, 3H, Ar–OCH3), 3.43 (d, J = 17.4 Hz, 1H, C1–He), 2.43 (d, J = 16.5 Hz, 1H, C1–Ha), 2.27 − 2.19 (m, 2H), 2.11 − 2.02 (m, 2H), 1.94 − 1.89 (m, 1H), 1.84 − 1.69 (m, 4H), 1.58 − 1.52 (m, 4H), 1.46 − 1.27 (m, 5H), 1.20 − 1.15 (m, 9H), 1.07 − 0.96 (m, 7H), 0.93 − 0.91 (m, 3H), 0.88 (s, 3H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 207.51, 183.73, 150.77, 143.93, 138.04, 134.74, 130.18, 126.93, 125.92, 125.70, 125.05, 124.74, 121.41, 112.32, 55.92, 52.96, 52.76, 48.12, 45.27, 45.18, 45.06, 42.24, 39.37, 39.15, 38.84, 36.70, 35.94, 32.12, 30.66, 29.77, 28.01, 24.13, 23.71, 23.44, 22.54, 21.15, 20.34, 17.04, 16.73, 15.82. ESI-HRMS (m/z): calculated for C40H54N3O4+ [M + H]+: 640.4109, found: 640.4106.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS,E)-1,2,6a,6b,9,9,12a-heptamethyl-10-oxo-11-((1–(3,4,5-trimethoxyphenyl)-1H-1,2,3-triazol-4-yl)methylene)-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylic acid (1j)
White solid; yield, 76%; m.p. >250 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.04 (s, 1H, triazole-H), 7.50 (s, 1H, –CO–C = CH–), 6.99 (s, 2H, Ar–H), 5.33 (s, 1H, C12–H), 3.97 − 3.92 (m, 9H, Ar–OCH3), 3.55 (d, J = 17.7 Hz, 1H, C1–He), 2.48 (d, J = 17.4 Hz, 1H, C1–Ha), 2.26 − 2.19 (m, 2H), 2.10 − 2.01 (m, 2H), 1.94 − 1.89 (m, 1H), 1.83 − 1.69 (m, 4H), 1.58 − 1.52 (m, 4H), 1.45 − 1.27 (m, 5H), 1.19 − 1.15 (m, 9H), 1.06 − 0.91 (m, 10H), 0.87 (s, 3H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 207.63, 183.75, 154.01(2C), 145.15, 138.56, 137.93, 135.76, 132.46, 125.75, 123.49, 123.21, 98.44(2C), 61.07, 56.45(2C), 53.00, 52.73, 48.11, 45.17, 45.10, 45.06, 42.22, 39.37, 39.17, 38.84, 36.71, 35.96, 32.10, 30.65, 29.74, 28.01, 24.12, 23.72, 23.44, 22.58, 21.15, 20.36, 17.05, 16.74, 15.78. ESI-HRMS (m/z): calculated for C42H58N3O6+ [M + H]+: 700.4320, found: 700.4318.
2.4. General procedure for synthesis of compound (2a–2e)
A mixture of UA (91.2 mg, 0.20 mmol), K2CO3 (41.5 mg, 0.30 mmol) and different phenyl 1,2,3-triazole chloroacetamides (0.21 mmol) in CH3CN (15 ml) was stirred at 60 °C for 2–3 h. After confirming the reaction progress by TLC, the solvent was evaporated in vacuo. The mixture was dissolved in 15 ml ethyl acetate, and then washed thrice with saline (5 ml). The final products were purified using preparative HPLC equipped with a C-18 column. A gradient elution was performed with tetrahydrofuran and water as the mobile phase and monitored at 220 nm and 254 nm.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-2-oxo-2-((1-phenyl-1H-1,2,3-triazol-4-yl)methylamino)ethyl-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (2a)
White powder; yield, 80%; m.p. 170–172 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.04 (s, 1H, triazole-H), 7.76 (d, J = 7.8 Hz, 2H, Ar–H), 7.57 − 7.44 (m, 3H, Ar–H), 6.88 (t, J = 6.0 Hz, 1H, –CO–NH–), 5.27 (s, 1H, C12–H), 4.75 − 4.68 (m, 3H, –CO–O–CH2–, –CO–NH–CHe), 4.40 (d, J = 15.6 Hz, 1H, –CO–NH–CHa), 3.22 − 3.17 (m, 1H, C3–OH), 2.24 (d, J = 11.4 Hz, 1H), 2.12 − 2.02 (m, 1H), 1.93 − 1.84 (m, 1H), 1.75 − 1.67 (m, 4H), 1.64 − 1.62 (m, 2H), 1.58 − 1.44 (m, 6H), 1.41 − 1.22 (m, 6H), 1.15 − 1.13 (m, 1H), 1.09 (s, 3H), 0.98 − 0.97 (m, 7H), 0.90 − 0.88 (m, 4H), 0.77 − 0.74 (m, 5H), 0.70 − 0.66 (m, 1H), 0.63 (s, 3H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 176.02, 167.81, 144.81, 139.23, 136.82, 129.82(2C), 128.99, 125.45, 120.72, 120.38(2C), 78.94, 62.82, 55.11, 52.90, 48.42, 47.37, 42.15, 39.43, 39.14, 38.78, 38.70, 38.44, 36.85, 36.70, 34.52, 32.77, 30.51, 28.10, 27.84, 27.13, 24.42, 23.62, 23.08, 21.10, 18.20, 16.98, 16.89, 15.57, 15.26. ESI-HRMS (m/z): calculated for C41H59N4O4+ [M + H]+: 671.4531, found: 671.4528.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-2-((1–(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methylamino)-2-oxoethyl-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (2b)
White powder; yield, 82%; m.p. 176–178 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.02 (s, 1H, triazole-H), 7.72 (d, J = 8.7 Hz, 2H, Ar–H), 7.53 (d, J = 8.7 Hz, 2H, Ar–H), 6.88 (t, J = 6.0 Hz, 1H, –CO–NH–), 5.28 (s, 1H, C12–H), 4.75 − 4.66 (m, 3H, –CO–O–CH2–, –CO–NH–CHe), 4.39 (d, J = 15.9 Hz, 1H, –CO–NH–CHa), 3.23 − 3.18 (m, 1H, C3–OH), 2.24 (d, J = 11.1 Hz, 1H), 2.13 − 2.03 (m, 1H), 1.96 − 1.86 (m, 1H), 1.80 − 1.67 (m, 5H), 1.64 − 1.46 (m, 8H), 1.42 − 1.24 (m, 6H), 1.16 − 1.13 (m, 1H), 1.09 (s, 3H), 1.01 − 0.94 (m, 8H), 0.90 − 0.88 (m, 3H), 0.82 − 0.79 (m, 3H), 0.75 (s, 2H), 0.71 − 0.67 (m, 1H), 0.63 (s, 2H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 176.04, 167.96, 143.16, 139.26, 135.24, 134.94, 130.04(2C), 125.43, 121.59(2C), 120.89, 78.95, 62.77, 55.11, 52.91, 48.42, 47.38, 42.17, 39.44, 39.14, 38.78, 38.70, 38.48, 36.88, 36.70, 34.42, 32.79, 30.51, 28.10, 27.85, 27.13, 24.42, 23.62, 23.13, 21.11, 18.22, 16.98, 16.92, 15.58, 15.29. ESI-HRMS (m/z): calculated for C41H58ClN4O4+ [M + H]+: 705.4141, found: 705.4146.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-2-((1–(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methylamino)-2-oxoethyl-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (2c)
White powder; yield, 80%; m.p. 180–181 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.94 (s, 1H, triazole-H), 7.65 (d, J = 9.0 Hz, 2H, Ar–H), 7.04 (d, J = 9.0 Hz, 2H, Ar–H), 6.87 (t, J = 6.0 Hz, 1H, –CO–NH–), 5.27 (s, 1H, C12–H), 4.74 − 4.66 (m, 3H, –CO–O–CH2–, –CO–NH–CHe), 4.40 (d, J = 15.9 Hz, 1H, –CO–NH–CHa), 3.89 (s, 3H, ph–OCH3), 3.23 − 3.17 (m, 1H, C3–OH), 2.24 (d, J = 10.5 Hz, 1H), 2.09 − 2.03 (m, 1H), 1.93 − 1.85 (m, 1H), 1.75 − 1.68 (m, 4H), 1.60 − 1.45 (m, 9H), 1.42 − 1.27 (m, 6H), 1.15 − 1.13 (m, 1H), 1.09 (s, 3H), 1.00 − 0.98 (m, 7H), 0.90 − 0.88 (m, 3H), 0.80 (s, 3H), 0.75 (s, 3H), 0.71 − 0.67 (m, 1H), 0.63 (s, 2H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 176.03, 167.82, 160.02, 144.53, 139.15, 130.21, 125.47, 122.02(2C), 120.88, 114.83(2C), 78.93, 62.79, 55.65, 55.12, 52.88, 48.40, 47.38, 42.14, 39.43, 39.13, 38.77, 38.70, 38.46, 36.87, 36.69, 34.49, 32.78, 30.51, 28.10, 27.85, 27.15, 24.41, 23.61, 23.11, 21.10, 18.20, 16.97, 16.90, 15.59, 15.29. ESI-HRMS (m/z): calculated for C42H61N4O5+ [M + H]+: 701.4636, found: 701.4640.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-2-oxo-2-((1-p-tolyl-1H-1,2,3-triazol-4-yl)methylamino)ethyl-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (2d)
White powder; yield, 81%; m.p. 186–187 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 7.99 (s, 1H, triazole-H), 7.62 (d, J = 8.4 Hz, 2H, Ar–H), 7.33 (d, J = 8.4 Hz, 2H, Ar–H), 6.88 (t, J = 5.7 Hz, 1H, –CO–NH–), 5.26 (s, 1H, C12–H), 4.73 − 4.66 (m, 3H, –CO–O–CH2–, –CO–NH–CHe), 4.40 (d, J = 15.9 Hz, 1H, –CO–NH–CHa), 3.22 − 3.18 (m, 1H, C3–OH), 2.43 (s, 3H, ph–CH3), 2.24 (d, J = 11.1 Hz, 1H), 2.12–2.02 (m, 1H), 1.93 − 1.83 (m, 1H), 1.76 − 1.66 (m, 5H), 1.63 − 1.45 (m, 7H), 1.39 − 1.21 (m, 6H), 1.13 − 1.12 (m, 1H), 1.08 (s, 3H), 1.03 − 0.97 (m, 7H), 0.89 − 0.87 (m, 4H), 0.78 (s, 3H), 0.74 (s, 3H), 0.70 − 0.66 (m, 1H), 0.62 (s, 2H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 175.99, 167.73, 144.76, 139.19, 139.00, 134.62, 130.26(2C), 125.46, 120.50, 120.26(2C), 78.92, 62.81, 55.11, 52.89, 48.40, 47.36, 42.14, 39.42, 39.13, 38.77, 38.69, 38.44, 36.85, 36.69, 34.63, 32.76, 30.50, 28.09, 27.82, 27.14, 24.42, 23.61, 23.08, 21.09(2C), 18.19, 16.97, 16.88, 15.55, 15.26. ESI-HRMS (m/z): calculated for C42H61N4O4+ [M + H]+: 685.4687, found: 685.4685.
(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-2-((1–(3,4-dichlorophenyl)-1H-1,2,3-triazol-4-yl)methylamino)-2-oxoethyl-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate (2e)
White powder; yield, 86%; m.p. 203–204 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.03 (s, 1H, triazole-H), 7.93 (s, 1H, Ar–H), 7.63 (s, 2H, Ar–H), 6.88 (t, J = 6.3 Hz, 1H, –CO–NH–), 5.28 (s, 1H, C12–H), 4.76 − 4.66 (m, 3H, –CO–O–CH2–, –CO–NH–CHe), 4.39 (d, J = 15.9 Hz, 1H, –CO–NH–CHa), 3.23 − 3.18 (m, 1H, C3–OH), 2.24 (d, J = 11.1 Hz, 1H), 2.13–2.03 (m, 1H), 1.95 − 1.87 (m, 1H), 1.80 − 1.66 (m, 4H), 1.63 − 1.60 (m, 4H), 1.53 − 1.47 (m, 4H), 1.42 − 1.23 (m, 7H), 1.14 − 1.10 (m, 4H), 1.03 − 0.98 (m, 6H), 1.08 (s, 3H), 0.90 − 0.85 (m, 5H), 0.80 − 0.76 (m, 5H), 0.72 − 0.68 (m, 1H), 0.63 (s, 2H), 0.70 − 0.66 (m, 1H), 0.62 (s, 2H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 175.99, 167.91, 145.51, 139.39, 135.90, 134.12, 133.03, 131.50, 125.38, 122.17, 120.58, 119.23, 78.92, 62.79, 55.10, 52.94, 48.43, 47.37, 42.19, 39.44, 39.14, 38.81, 38.70, 38.49, 36.88, 36.72, 34.59, 32.78, 30.49, 28.09, 27.82, 27.15, 24.42, 23.62, 23.13, 21.09, 18.21, 16.98, 16.93, 15.56, 15.28. ESI-HRMS (m/z): calculated for C41H57Cl2N4O4+ [M + H]+: 739.3751, found: 739.3753.
2.5. General procedure for synthesis of compound (3a–3e)
A mixture of UA (91.2 mg, 0.20 mmol), K2CO3 (41.5 mg, 0.30 mmol) and different 4–(2-chloroethyl)-3-phenyl-4H-1,2,4-triazoles (0.21 mmol) in CH3CN (15 ml) was stirred at 60 °C for 4–6 h. After confirming the reaction progress by TLC, the solvent was evaporated in vacuo. The mixture was dissolved in 15 ml ethyl acetate, and then washed thrice with saline (5 ml). Final products were purified by preparative HPLC equipped with a C-18 column. A gradient elution was performed with tetrahydrofuran and water as the mobile phase and monitored at 220 nm and 254 nm.
2. –(3-phenyl-4H-1,2,4-triazol-4-yl)ethyl(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylate (3a)
White powder; yield, 77%; m.p. 243–244 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.33 (s, 1H, triazole-H), 7.64 − 7.54 (m, 5H, Ar–H), 5.17 (s, 1H, C12–H), 4.31 − 4.24 (m, 4H, –O–CH2CH2–N–), 3.24 − 3.21 (m, 1H, C3–OH), 2.16 (d, J = 11.7 Hz, 1H), 2.08 − 1.86 (m, 4H), 1.65 − 1.61 (m, 6H), 1.53 − 1.44 (m, 5H), 1.40 − 1.27 (m, 5H), 1.07 − 1.05 (m, 4H), 1.00 − 0.97 (m, 7H), 0.91 (s, 3H), 0.85 (d, J = 6.3 Hz, 3H), 0.79 (s, 3H), 0.72 (d, J = 10.2 Hz, 1H), 0.63 (s, 3H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 177.06, 153.94, 144.18, 137.82, 130.34, 129.07 (2C), 128.97 (2C), 126.56, 125.97, 78.96, 62.39, 55.16, 52.88, 48.28, 47.44, 44.00, 41.98, 39.48, 39.00, 38.85, 38.73, 38.55, 36.95, 36.68, 32.85, 30.46, 28.13, 27.89, 27.20, 24.22, 23.58, 23.23, 21.09, 18.27, 17.01, 16.96, 15.62, 15.41. ESI-HRMS (m/z): calculated for C40H58N3O3+ [M + H]+: 628.4473, found: 628.4470.
2. –(3-(4-chlorophenyl)-4H-1,2,4-triazol-4-yl)ethyl(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylate (3b)
White powder; yield, 79%; m.p. 245–246 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.32 (s, 1H, triazole-H), 7.61 (d, J = 8.7 Hz, 2H, Ar–H), 7.53 (d, J = 8.7 Hz, 2H, Ar–H), 5.16 (s, 1H, C12–H), 4.29 − 4.25 (m, 4H, –O–CH2CH2–N–), 3.24 − 3.19 (m, 1H, C3–OH), 2.14 (d, J = 11.1 Hz, 1H), 2.04–1.95 (m, 1H), 1.93 − 1.81 (m, 2H), 1.69 − 1.66 (m, 3H), 1.63 − 1.55 (m, 6H), 1.51 − 1.43 (m, 4H), 1.36 − 1.27 (m, 5H), 1.07 − 1.04 (m, 4H), 1.00 − 0.96 (m, 6H), 0.91 (s, 3H), 0.86 − 0.84 (m, 3H), 0.79 (s, 3H), 0.71 (d, J = 11.7 Hz, 1H), 0.61 (s, 3H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 177.00, 144.33, 144.29, 137.80, 137.07, 130.30(2C), 129.52(2C), 125.97, 124.43, 78.96, 62.12, 55.16, 52.89, 48.30, 47.42, 44.33, 41.99, 39.48, 38.99, 38.85, 38.73, 38.54, 36.94, 36.69, 32.84, 30.43, 28.13, 27.88, 27.19, 24.22, 23.57, 23.22, 21.08, 18.27, 17.02, 16.96, 15.62, 15.41. ESI-HRMS (m/z): calculated for C40H57ClN3O3+ [M + H]+: 662.4083, found: 662.4081.
2. –(3-(4-fluorophenyl)-4H-1,2,4-triazol-4-yl)ethyl(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylate (3c)
White powder; yield, 85%; m.p. 221–222 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.32 (s, 1H, triazole-H), 7.68–7.63 (m, 2H, Ar–H), 7.27–7.22 (m, 2H, Ar–H), 5.17 (s, 1H, C12–H), 4.28–4.25 (m, 4H, –O–CH2CH2–N–), 3.25 − 3.21 (m, 1H, C3–OH), 2.16 (d, J = 11.1 Hz, 1H), 2.04 − 1.98 (m, 1H), 1.91 − 1.85 (m, 2H), 1.69 − 1.63 (m, 4H), 1.56 − 1.43 (m, 7H), 1.36 − 1.25 (m, 6H), 1.08 − 1.05 (m, 4H), 1.00 − 0.97 (m, 7H), 0.91 (s, 3H), 0.87 − 0.84 (m, 3H), 0.79 (s, 3H), 0.72 (d, J = 10.5 Hz, 1H), 0.62 (s, 3H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 177.00, 160.28, 153.00, 144.16, 137.81, 131.25, 130.14, 125.97, 122.05, 116.62, 116.32, 78.94, 62.14, 55.16, 52.88, 48.30, 47.42, 44.18, 41.99, 39.48, 38.99, 38.85, 38.73, 38.54, 36.94, 36.70, 32.85, 30.43, 28.13, 27.87, 27.19, 24.22, 23.57, 23.22, 21.07, 18.26, 17.01, 16.96, 15.62, 15.40. ESI-HRMS (m/z): calculated for C40H57FN3O3+ [M + H]+: 646.4378, found: 646.4373.
2. –(3-(4-nitrophenyl)-4H-1,2,4-triazol-4-yl)ethyl(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylate (3d)
White powder; yield, 82%; m.p. >250 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.43 − 8.39 (m, 3H, triazole-H, Ar–H), 7.93 (d, J = 8.7 Hz, 2H, Ar–H), 5.15 (s, 1H, C12–H), 4.33 (dd, J = 17.7, 5.1 Hz, 4H, –O–CH2CH2–N–), 3.24 − 3.19 (m, 1H, C3–OH), 2.13 (d, J = 11.1 Hz, 1H), 2.07 − 1.97 (m, 1H), 1.93 − 1.77 (m, 2H), 1.63 − 1.59 (m, 5H), 1.56 − 1.45 (m, 6H), 1.35 − 1.25 (m, 6H), 1.07 (s, 4H), 1.00 − 0.96 (m, 7H), 0.90 (s, 3H), 0.86 − 0.84 (m, 3H), 0.79 (s, 3H), 0.71 (d, J = 11.1 Hz, 1H), 0.60 (s, 3H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 176.97, 151.99, 148.83, 144.91, 137.76, 132.67, 129.82(2C), 126.00, 124.28(2C), 78.92, 62.08, 55.13, 52.90, 48.33, 47.39, 44.28, 41.98, 39.47, 38.97, 38.87, 38.72, 38.52, 36.92, 36.72, 32.82, 30.40, 28.12, 27.86, 27.16, 24.24, 23.56, 23.19, 21.06, 18.24, 17.02, 16.96, 15.62, 15.39. ESI-HRMS (m/z): calculated for C40H57N4O5+ [M + H]+: 673.4323, found: 673.4320.
2. –(3-(4-methoxyphenyl)-4H-1,2,4-triazol-4-yl)ethyl(1S,2R,4aS,6aS,6bR,8aR,12aR,12bR,14bS)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-octadecahydropicene-4a(2H)-carboxylate (3e)
White powder; yield, 76%; m.p. 233 − 235 °C; 1H-NMR (CDCl3, 300 MHz, ppm): δ 8.29 (s, 1H, triazole-H), 7.57 (d, J = 9.0 Hz, 2H, Ar–H), 7.04 (d, J = 8.7 Hz, 2H, Ar–H), 5.17 (s, 1H, C12–H), 4.28 − 4.24 (m, 4H, –O–CH2CH2–N–), 3.89 (s, 3H, ph-OCH3), 3.24 − 3.19 (m, 1H, C3–OH), 2.16 (d, J = 11.1 Hz, 1H), 2.03 − 1.97 (m, 1H), 1.91 − 1.84 (m, 2H), 1.66 − 1.56 (m, 7H), 1.52 − 1.43 (m, 5H), 1.40 − 1.25 (m, 6H), 1.07 (s, 4H), 1.00 − 0.96 (m, 6H), 0.91 (s, 3H), 0.86 − 0.84 (m, 3H), 0.79 (s, 3H), 0.72 (d, J = 11.4 Hz, 1H), 0.62 (s, 3H). 13C-NMR (CDCl3, 75 MHz, ppm): δ 176.98, 161.89, 152.04, 143.85, 137.84, 133.86, 130.79(2C), 125.93, 114.81(2C), 78.97, 61.95, 55.51, 55.15, 52.85, 48.29, 47.42, 44.17, 41.98, 39.46, 38.99, 38.81, 38.73, 38.54, 36.94, 36.67, 32.84, 30.43, 28.13, 27.87, 27.20, 24.21, 23.56, 23.21, 21.08, 18.26, 17.02, 16.95, 15.63, 15.41. ESI-HRMS (m/z): calculated for C41H60N3O4+ [M + H]+: 658.4578, found: 658.4580.
2.6. In vitro anti-T. gondii activity
The cytotoxicity of compounds was determined using the previously published thiazolyl blue-based colorimetric method. For this, HeLa cells were used as host cells and their ability to resist invasion by T. gondii RH strain tachyzoites in vitro was checked. The cells were plated in 96-well plates at an appropriate density to ensure exponential growth throughout the experimental period (3 × 103 cells per well) and then allowed to adhere for 24 h at 37 °C. The cells were infected with T. gondii (1.5 × 104 tachyzoites/well), followed by incubation for 24 h. All compounds were prepared in dimethyl sulfoxide (DMSO) at a stock concentration of 100 mM. Serial dilutions (1–1000 μM) of each compound were tested. Spiramycin was used as a positive control. After 24 h of incubation, 10 μL of MTT solution were added to each well and cells were incubated for a further 2 h. The optical density (OD) was read on a microplate reader at a wavelength of 492 nm. The IC50 in HeLa cells, IC50 in T. gondii and selectivity index were calculated using Microsoft Excel.
2.7. In vivo anti-T. gondii activity
Thirty female KM mice were used to establish an animal model of acute T. gondii infection. These were randomly divided into five groups: infected untreated, normal, infected with spiramycin treatment, infected with 1e treatment and infected with 3d treatment. Each group consisted of six mice. Four hours after infection, 100 mg/kg of the compounds was administered to the mice by gavage, once a day for 4 consecutive days, whereas the untreated group was administered the same dose of physiological saline. On the fifth day, blood from the eyes of mice was collected and they were sacrificed by cervical dislocation. Their abdominal cavity was rinsed with sterile physiological saline to collect the parasites/tachyzoites. These were counted under the light microscope, and the inhibition rate of parasites was calculated. The liver and spleen were dissected and liver and spleen indexes, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and liver homogenate glutathione (GSH) and malonaldehyde (MDA) were determined.
2.8. Molecular docking
Molecular docking was performed using the Discovery Studio (DS) 2017 software. The protein and ligand samples were prepared, water molecules were deleted, and a DS Server added hydrogen. The docking result was treated with DS Client. In this study, three crystal structures of the proteins were selected for docking, PDB ID: 6BFA (calcium-dependent protein kinase 1)21, 1LII (adenosine kinase)22 and 3MB8 (purine nucleoside phosphorylase)23. The different xyz coordinates and radii of these proteins were defined as the binding site spheres. The output poses of the ligands generated were analysed using the LibDockScore function to find out the best complimentary match between the ligand and the receptor. The protocol, CDOCKER was used to perform the docking.
3. Results and discussion
3.1. Chemistry
Scheme 2 shows the procedure adopted to obtain target compounds. UA-1 was obtained by Jones oxidation of UA at 0 °C. Compounds 1a–1j were prepared by Claisen Schmidt condensation of UA-1 with different aldehydes in the presence of ethanolic KOH at 30 °C. Good yields (76–82%) were obtained with this method. All other UA derivatives (2a–2e and 3a–3e) were synthesised from various chlorinated derivatives via nucleophilic substitution in good to excellent yields (76–86%). Before biological evaluation, all target compounds were characterised via HRMS, 1H-NMR and 13C-NMR.
Scheme 2.
Reagents and conditions: (a) Ia–Ij, KOH, CH3CH2OH, 30 °C. (b) IIa–IIe, IIIa–IIIe, K2CO3, CH3CN, 60 °C.
3.2. Evaluation of anti-T. gondii activity in vitro and preliminary structure-activity relationship
Selectivity index is a measure of specific resistance to T. gondii. As shown in Table 1, the SI value of the lead compound UA (0.62) was lower than that of the positive control drug spiramycin (0.72), indicating a certain degree of anti-T. gondii activity of UA. Among UA derivatives, nine compounds exhibited higher anti-T. gondii activity than UA alone (1e, 1f, 1g, 1h, 1j, 3a, 3c, 3d and 3e), and eight compounds exhibited an activity higher than spiramycin (1e, 1f, 1g, 1h, 1j, 3a, 3c and 3d). Besides, with the exception of compound 3b, the IC50 value of all other compounds was higher than that of UA, indicating these compounds to be less cytotoxic than UA. Similarly, compounds 1e, 1f, 1g, 1h, 1j and 3d displayed a higher anti-T. gondii activity and less cytotoxicity when compared with spiramycin.
Table 1.
In vitro T. gondii growth inhibition and cytotoxicity on HeLa cells.
| Compounds | R | IC50a in HeLa cells (μM) | IC50b in T. gondii (μM) | SIc |
|---|---|---|---|---|
| 1a | -H | >1000 | >1000 | – |
| 1b | 2-F | 419.6 | 711.9 | 0.59 |
| 1c | 3-F | >1000 | >1000 | – |
| 1d | 4-F | >1000 | >1000 | – |
| 1e | 2-Cl | 466.1 | 239.6 | 1.95 |
| 1f | 3,4-Cl | 408.0 | 462.1 | 0.88 |
| 1g | 2-Br | 240.4 | 301.7 | 0.80 |
| 1h | 2-I | 230.4 | 273.8 | 0.84 |
| 1i | 2-OCH3 | >1000 | >1000 | – |
| 1j | 3,4,5-OCH3 | 353.7 | 301.3 | 1.17 |
| 2a | -H | 836.8 | >1000 | – |
| 2b | 4-Cl | 328.7 | >1000 | – |
| 2c | 4-OCH3 | >1000 | >1000 | – |
| 2d | 4-CH3 | >1000 | >1000 | – |
| 2e | 3,4-Cl | >1000 | >1000 | – |
| 3a | -H | 101.5 | 88.0 | 1.15 |
| 3b | 4-Cl | 2.4 | 6.7 | 0.36 |
| 3c | 4-F | 88.2 | 61.4 | 1.44 |
| 3d | 4-NO2 | 226.7 | 128.0 | 1.77 |
| 3e | 4-OCH3 | 78.2 | 116.5 | 0.67 |
| Spiramycin | – | 189.0 | 262.2 | 0.72 |
| Ursolic Acid | – | 44.8 | 72.2 | 0.62 |
IC50 in HeLa cells: Median toxicity dose, a measure of cytotoxicity against host cells.
IC50 in T. gondii: Median inhibitory concentration, a measure of tachyzoite inhibition.
SI: Selectivity index, a measure of efficacy, calculated by IC50 in HeLa cells/IC50 in T. gondii.
Compounds 1a–1j are products of a reaction between UA-1 and Ia–Ij. The anti-T. gondii activity of these compounds with different substitutions on the benzene ring was found to be in the following order: 2-Cl > 3,4,5-OCH3 > 3,4-Cl > 2-I > 2-Br > 2-F > 2-OCH3=H. Based on an overall comparison, we hypothesised that introduction of halogen substituents at the ortho position and electron-donating group at the 3,4,5-position of benzene ring could improve the anti-T. gondii activity. Compounds 2a–2e were generated from UA and IIa–IIe. Unfortunately, all of these compounds lost their anti-T. gondii activity. Among the compounds 3a–3e, which react with IIIa–IIIe, four compounds showed considerably higher anti-T. gondii activity. It seems that the anti-T. gondii ability was enhanced after the introduction of strong electron-withdrawing group (–F, –NO2) to the para position of the benzene ring. Based on these findings, we decided to conduct an in-depth study of anti-T. gondii activity of compounds 1e and 3d in mice, owing to their strong anti-T. gondii activity in vitro.
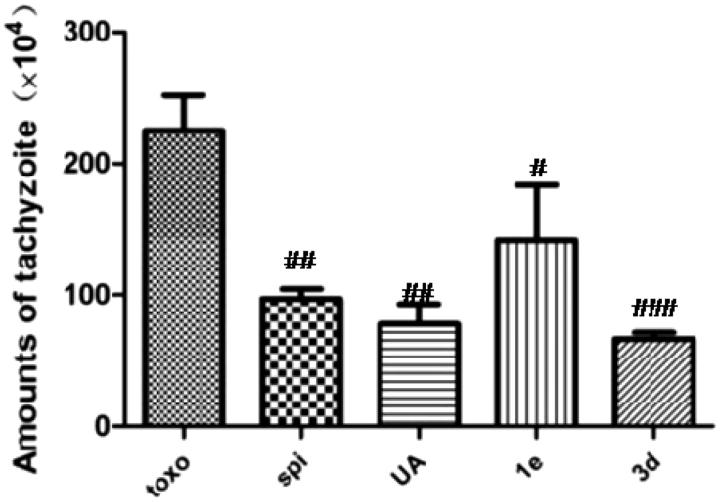
3.3. Number of tachyzoites in vivo
As shown in Table 2 and Figure 2, the number of intraperitoneal tachyzoites in untreated KM mice was 225 × 104. After treatment with 100 mg/kg of different compounds, this number decreased to varying degrees in the ascitic fluid of spiramycin-, UA-, compound 1e- and compound 3d-treated mice, with inhibitory rates being 56.8%, 65.4%, 37.0% and 70.4%, respectively. It is clearly interpreted from these data that treatment with compound 3d could significantly decrease the number of tachyzoites in T. gondii-infected KM mice (p < .001). It even showed better anti-T. gondii activity than spiramycin and UAin vivo.
Table 2.
In vivo anti-T. gondii activity.
T. gondii-infected KM mice with no treatment.
Spiramycin.
Figure 2.
Effect of compounds on the number of tachyzoites in KM mice, n = 6, #p < .05, ##p < .01, ###p < .001 compared with toxo group.
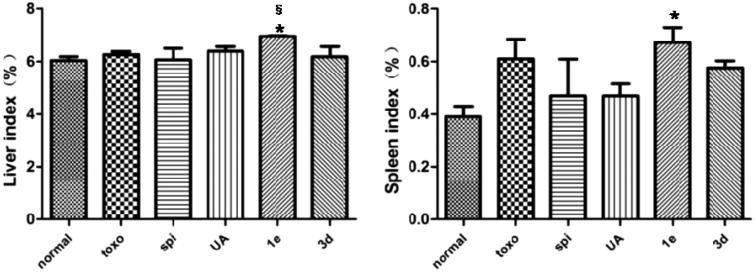
3.4. Liver and spleen indexes
Liver and spleen indexes were used to evaluate the protective effect of drugs on viscera. As shown in Figure 3, compared with the normal group, the liver index of the mice infected with T. gondii increased only slightly. Although a significant increase in the spleen index was observed in mice infected with T. gondii, this increase was subjugated by treatment with spiramycin, UA or compound 3d. However, changes in spleen index did not show any statistically significant differences.
Figure 3.
Effect of compounds on liver and spleen weights in T. gondii-infected KM mice, *p < .05 compared with normal group; §p < .05 compared with spi group.
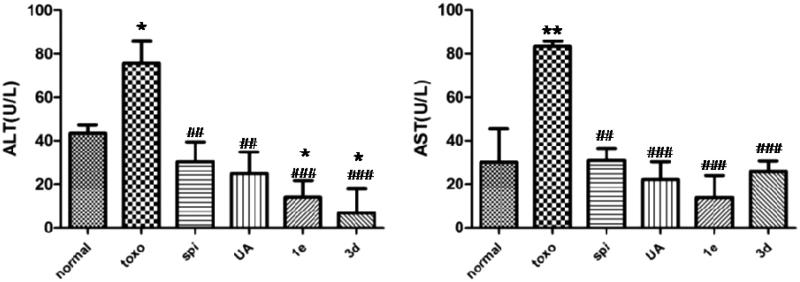
3.5. ALT and AST
Levels of serum ALT and AST act as indicators of hepatotoxicity. To further study the toxicity of these compounds, ALT and AST levels in the serum of KM mice after infection with T. gondii were measured (Figure 4). T. gondii infection resulted in a significant elevation of serum ALT and AST levels as compared with the normal group. Treatment with UA, 1e and 3d led to a striking reduction in these levels as compared with the untreated group. These results indicated UA, 1e and 3d could provide resistance against T. gondii-mediated hepatotoxicity.
Figure 4.
Effect of compounds on ALT and AST levels in T. gondii-infected KM mice, *p < .05, **p < .01 compared with normal group; ##p < .01, ###p < .001 compared with toxo group.
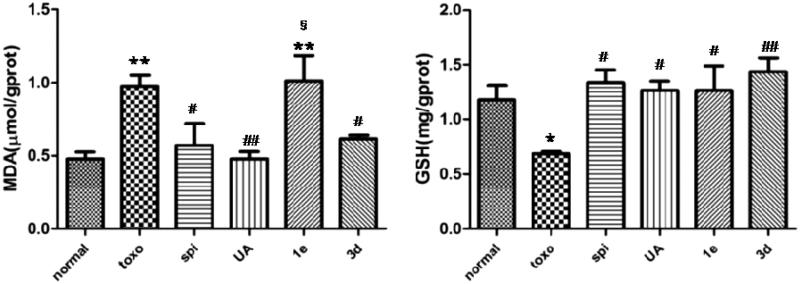
3.6. MDA and GSH
Free radicals generated within cells cause peroxidation of lipids, resulting in the formation of MDA, which, in turn, causes cross-linking and polymerisation of proteins, nucleic acids and other macromolecules, thereby exerting cytotoxicity. As can be seen from the data in Figure 5, the untreated group had a higher MDA content compared with the normal group (p < .01), whereas levels of MDA significantly decreased after treatment with spiramycin, UA or compound 3d. GSH is an important antioxidant that scavenges the free radicals in the body. It combines with free radicals and heavy metals, thereby converting them to harmless substances that are excreted from the body24. Compared with the normal group, the GSH content in the untreated group was significantly decreased (p < .05). However, compounds 3d and 1e could significantly increase the GSH content as compared to the untreated group, and had a similar efficacy to UA and spiramycin. These results implied that the anti-oxidative effects of UA and compound 3d were comparable to that of spiramycin.
Figure 5.
Effect of compounds on MDA and GSH levels in T. gondii-infected KM mice, *p < .05, **p < .01 compared with normal group; #p < .05, ##p < .01 compared with toxo group; §p < .05 compared with spi group.
3.7. Molecular docking analysis
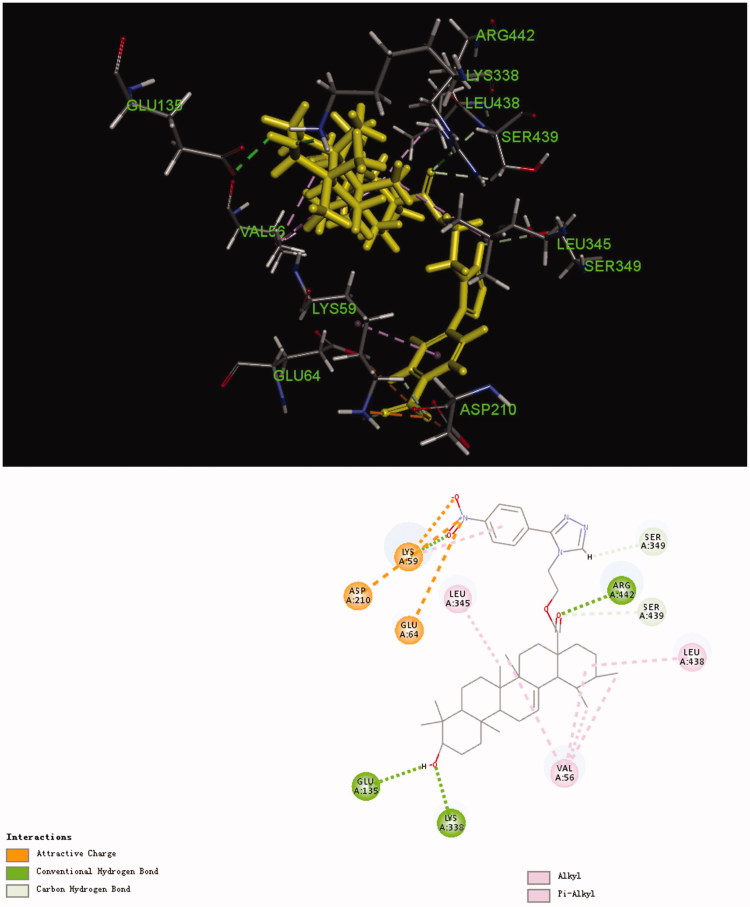
TgCDPK1 plays a crucial role in the motility and gliding of T. gondii, as well as the adenosine kinase and purine nucleoside phosphorylase are key purine metabolic enzymes from the T. gondii21–23. Our in vivo study revealed compound 3d to significantly inhibit the proliferation of tachyzoites in the abdominal cavity of KM mice. Therefore, these three enzymes related to T. gondii metabolism were selected for molecular docking study and to determine possible targets with their specific modes of action (Table 3). Interestingly, only TgCDPK1 (6BFA) could be docked and expressed a high binding energy for the ligand. The CDOCKER interaction energy of compound 3d was 58.0486, slightly higher than that of UA, which was consistent with the result of in vivo. Figure 6 illustrates the binding mode of compound 3d in its active site; it was held in the active pocket of TgCDPK1 through a combination of interactions with TgCDPK1. The nitro group of compound 3d interacted with the –NH3+ group of Lys-A59, –COOH group of Asp-A210 and –COOH group of Glu-A64 via three important attractive charges. These interactions may explain the strong anti-T. gondii activity exhibited by compound 3d in this series. Meanwhile, the carbonyl group of UA interacted with the = NH moiety of Arg-A442 and the –CH2– moiety of Ser-A439 via hydrogen and carbon–hydrogen bonds, respectively, whereas the –OH group of UA interacted with the –COOH group of Glu-A135 and the –NH2 group of Lys-A338 via two hydrogen bonds. In addition, the 1,2,4-triazole moiety formed one carbon–hydrogen bond with Ser-A439 residue. We also observed that UA entered into an alkyl interaction with amino acid residues Val-A56, Leu-A345 and Leu-A438.
Table 3.
Scores of UA and compound 3d docked to different enzymes.
| Enzyme (PDB ID) | CDOCKER interaction energy |
|
|---|---|---|
| UA | Compound 3d | |
| 6BFA | 54.6825 | 58.0486 |
| 1LII | No docking | No docking |
| 3MB8 | No docking | No docking |
Figure 6.
Computer modelling of compound 3d binding to calcium-dependent protein kinase 1 (6BFA). Compound 3d was coloured in yellow.
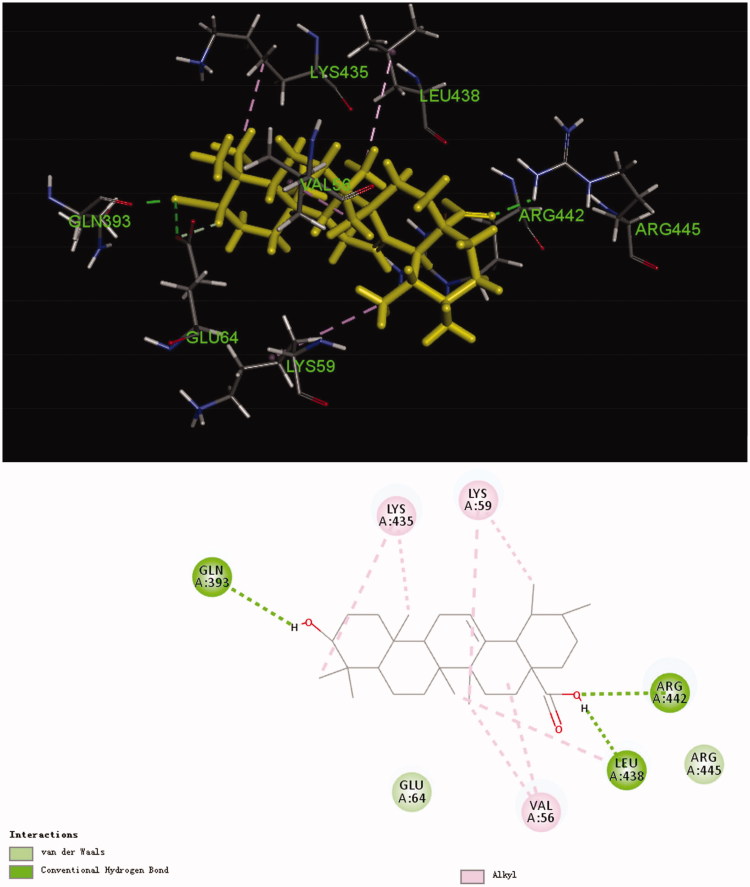
In order to better reflect the advantage of compound 3d, we also performed the molecular docking analysis of UA. As shown in Figure 7, three similar conventional hydrogen bonds are observed with residue Gln-A393, Leu-A438 and Arg-A442. However, compared with compound 3d, some significant chemical bonds such as attractive charges are missing. This may explain why compound 3d has better anti-T. gondii activity than UA. These results indicate compound 3d to possess a strong binding affinity for the enzyme and therefore could act as a possible TgCDPK1 inhibitor.
Figure 7.
Computer modelling of UA binding to calcium-dependent protein kinase 1 (6BFA). UA was coloured in yellow.
4. Conclusions
In the present study, 20 novel UA derivatives were synthesised and examined for their anti-T. gondii properties. Most of these compounds displayed some anti-T. gondii activity, with a less cytotoxicity than UAin vitro. The compound 3d exhibited the most potent anti-T. gondii activity in vivo and was superior to UA and spiramycin. Docking study confirmed the anti-T. gondii activity of 3d, as evident by the presence of three significant attractive charges and three hydrogen bonds in it that play a crucial role in its binding to the active site of TgCDPK1. Based on these findings, we conclude that compound 3d may serve as a potential candidate for developing effective and anti-T. gondii drugs with fewer side-effects.
Supplementary Material
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 21662036, 81160409 and 81260226).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Denkers EY, Schneider AG, Cohen SB, et al. . Phagocyte responses to protozoan infection and how Toxoplasma gondii meets the challenge. PLoS Pathog 2012;8:e1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mui EJ, Jacobus D, Milhous WK, et al. . Triazine inhibits Toxoplasma gondii tachyzoites in vitro and in vivo. Antimicrob Agents Chemother 2005;49:3463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Innes EA. Vaccination against Toxoplasma gondii: an increasing priority for collaborative research? Exp Rev Vaccine 2010;9:1117–19. [DOI] [PubMed] [Google Scholar]
- 4.D’Angelo JG, Bordo n. C ´Posner GH, et al. . Artemisinin derivatives inhibit Toxoplasma gondii in vitro at multiple steps in the lytic cycle. J Antimicrob Chemoth 2009;63:146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang HB, Shen QK, Wang H, et al. . Synthesis and evaluation of novel arctigenin derivatives as potential anti-Toxoplasma gondii agents. Eur J Med Chem 2018;158:414–27. [DOI] [PubMed] [Google Scholar]
- 6.Dar BA, Lone AM, Shah WA, et al. . Synthesis and screening of ursolic acid-benzylidine derivatives as potential anti-cancer agents. Eur J Med Chem 2016;111:26–32. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Meng Y, Zhao J, et al. . Synthesis and anti-tumor activity of novel amide derivatives of ursolic acid. Chem Res Chinese Universities 2008;24:42–6. [Google Scholar]
- 8.Wu PP, Zhang K, Lu YJ, et al. . In vitro and in vivo evaluation of the antidiabetic activity of ursolic acid derivatives. Eur J Med Chem 2014;80:502–8. [DOI] [PubMed] [Google Scholar]
- 9.Ma CM, Nakamura N, Hattori M, et al. . Inhibitory effects on HIV-1 protease of constituents from the wood of Xanthoceras sorbifolia. J Nat Prod 2000;63:238–42. [DOI] [PubMed] [Google Scholar]
- 10.Keita FT, Gasquet M, Giorgio CD, et al. . Antimalarial activity of four plants used in traditional medicine in Mali. Phytother Res 2000;14:45–7. [DOI] [PubMed] [Google Scholar]
- 11.Chattopadhyay D, Arunachalam G, Mandal AB, et al. . Antimicrobial and anti-inflammatory activity of folklore: Mallotus peltatus leaf extract. J Ethnopharmacol 2002;82:229–37. [DOI] [PubMed] [Google Scholar]
- 12.Wolska KI, Grudniak AM, Fiecek B, et al. . Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent Eur J Biol 2010;5:543–53. [Google Scholar]
- 13.Choi WH, Lee IA. Evaluation of anti-Toxoplasma gondii effect of ursolic acid as a novel toxoplasmosis inhibitor. Pharmaceuticals 2018;11:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu MC, Yang SJ, Jin LH, et al. . Synthesis and cytotoxicity of novel ursolic acid derivatives containing an acyl piperazine moiety. Eur J Med Chem 2012;58:128–35. [DOI] [PubMed] [Google Scholar]
- 15.Rashid S, Dar BA, Majeed R, et al. . Synthesis and biological evaluation of ursolic acid-triazolyl derivatives as potential anti-cancer agents. Eur J Med Chem 2013;66:238–45. [DOI] [PubMed] [Google Scholar]
- 16.Stocks MJ, Cheshire DR, Reynolds R. Efficient and regiospecific one-pot synthesis of substituted 1,2,4-triazoles. Org Lett 2004;6:2969–71. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Shen QK, Zhang HJ, et al. . Design and synthesis of novel dehydroepiandrosterone analogues as potent antiproliferative agents. Molecules 2018;23:2243–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharling L, Liu X, Gollapalli DR, et al. . A screening pipeline for antiparasitic agents targeting cryptosporidium inosine monophosphate dehydrogenase. PLoS Neglect Trop D 2010;4:e794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzitko K, Paneth A, Plech T, et al. . Triazole-based compound as a candidate to develop novel medicines to treat toxoplasmosis. Antimicrob Agents Chemother 2014;58:7583–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luan T, Cao LH, Deng H, et al. . Design and synthesis of C-19 isosteviol derivatives as potent and highly selective antiproliferative agents. Molecules 2018;24:121–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vidadala RSR, Rivas KL, Ojo KK, et al. . Development of an orally available and central nervous system (CNS) penetrant Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) Inhibitor with minimal human ether-a-go-go-related gene (hERG) activity for the treatment of toxoplasmosis. J Med Chem 2016;59:6531–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schumacher MA, Scott DM, Mathews II, et al. . Crystal structures of Toxoplasma gondii adenosine kinase reveal a novel catalytic mechanism and prodrug binding. J Mol Biol 2000;298:875–93. [DOI] [PubMed] [Google Scholar]
- 23.Donaldson TM, Cassera MB, Ho MC, et al. . Inhibition and structure of Toxoplasma gondii purine nucleoside phosphorylase. Eukaryot Cell 2014;13:572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi HJ, Yu ST, Lee KI, et al. . 6-Trifluoromethyl-2-thiouracil possesses anti-Toxoplasma gondii effect in vitro and in vivo with low hepatotoxicity. Exp Parasitol 2014;143:24–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.