Figure 2.
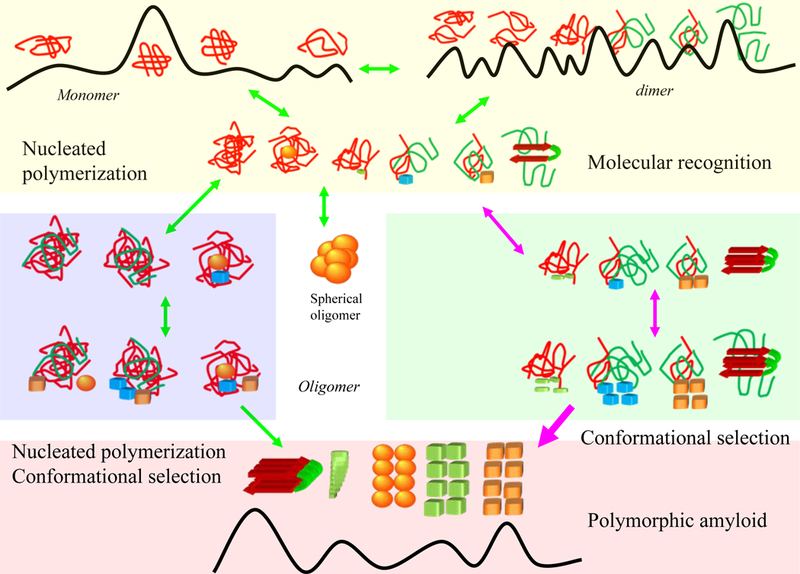
Conformational selection mechanism may operate in the early and late stages of amyloid polymerization. The top yellow box highlights that the initial perturbation or unfolding of the native protein structure can lead to the formation of small oligomers (top yellow box); the formation of nucleation site for further polymerization may take long time for total disordered monomers, with many rounds of association-disassociation events. Partially unfolded proteins, as in the case of domain swapping, may accelerate the formation through direct molecular recognition. The middle panels relate to the formation of larger oligomers. The blue box is the nucleation polymerization pathway and the green box depicts molecular recognition. The middle panel also highlights the formation of spherical particles. In the bottom panel, molecular recognition dominates fibril growth, with possibly leading to polymorphic amyloid states. Molecular recognition takes place via mutual conformation selection and population shift.
