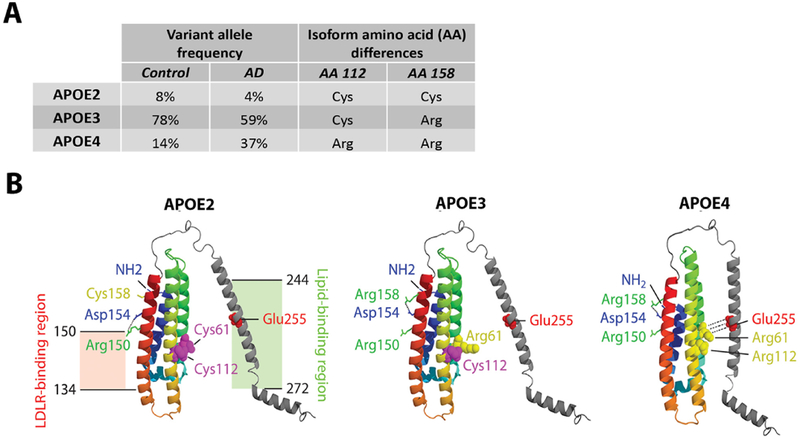
Figure 1. APOE isoforms, allele frequencies, and protein structures.
A) The three main APOE isoforms APOE2, APOE3, and APOE4, respectively encoded by the Apolipoprotein E2, E3, and E4 alleles, are the result of non-synonymous polymorphisms that cause amino acid changes at position 112 and 158 of the APOE protein (Rall et al., 1982; Weisgraber et al., 1981). APOE3 is the most common variant in the general population. The APOE4 variant is a major genetic risk factor for AD, while APOE2 is protective (Farrer et al., 1997). B) Structural models of lipid-free APOE are shown for each major isoform, based on X-ray crystallography, structure prediction, and circular dichroism spectroscopy (Zhong and Weisgraber, 2009). The N-terminal domain contains APOE’s low-density lipoprotein receptor (LDLR) region at amino acid residues 134 to 150, while the C-terminal holds the lipid-binding region at residues 244 to 272. Amino acid substitutions in APOE4 promote a salt-bridge between Arg61 and Glu255, which, compared to the APOE2 and APOE3 variants, drives increased domain interaction between the N- and C-terminal domains. In figure panel A, APOE allele frequencies are obtained, with permission, from American Medical Association © Farrer, L. et al. JAMA. 278, 1349–1356 (1997). Figure panel B is a reprint, with permission, from Annual Reviews © Yu, J. et al. Annu. Rev. Neurosci. 37, 79–100 (2014).