Supplemental Digital Content is Available in the Text.
A clear role of ICA69 in mediating the antihyperalgesic effects of electroacupuncture was confirmed, and the ICA69-PICK1-GluR2 molecular mechanism to explain these effects is proposed.
Keywords: ICA69, Electroacupuncture, Inflammatory pain, Protein interacting with C-kinase 1, GluR2
Abstract
Electroacupuncture (EA) is widely used in clinical settings to reduce inflammatory pain. Islet-cell autoantigen 69 (ICA69) has been reported to regulate long-lasting hyperalgesia in mice. ICA69 knockout led to reduced protein interacting with C-kinase 1 (PICK1) expression and increased glutamate receptor subunit 2 (GluR2) phosphorylation at Ser880 in spinal dorsal horn. In this study, we evaluated the role of ICA69 in the antihyperalgesic effects of EA and the underlying mechanism through regulation of GluR2 and PICK1 in spinal dorsal horn. Hyperalgesia was induced in mice with subcutaneous plantar injection of complete Freund adjuvant (CFA) to cause inflammatory pain. Electroacupuncture was then applied for 30 minutes every other day after CFA injection. When compared with CFA group, paw withdrawal frequency of CFA+EA group was significantly decreased. Remarkable increases in Ica1 mRNA expression and ICA69 protein levels on the ipsilateral side were detected in the CFA+EA group. ICA69 expression reached the peak value around day 3. More importantly, ICA69 deletion impaired the antihyperalgesic effects of EA on GluR2-p, but PICK1 deletion could not. Injecting ICA69 peptide into the intrathecal space of ICA69-knockout mice mimicked the effects of EA analgesic and inhibited GluR2-p. Electroacupuncture had no effects on the total protein of PICK1 and GluR2. And, EA could increase the formation of ICA69-PICK1 complexes and decrease the amount of PICK1-GluR2 complexes. Our findings indicate that ICA69 mediates the antihyperalgesic effects of EA on CFA-induced inflammatory pain by regulating spinal GluR2 through PICK1 in mice.
1. Introduction
Inflammatory pain is a severe clinical symptom that is difficult to treat and often leads to disabling of patients. Inflammation at the injury site was caused by tissue injury; then, the inflammatory mediators such as bradykinin, substance P, PGE2, ATP, IL-1, and IL-1β, which act on nerve terminal nociceptors, were released. They can alter the nociceptor excitability through transcriptional and/or posttranslational mechanisms, giving rise to hypersensitivity.25,31,36 Subcutaneous injection of inflammatory agents, such as formalin, capsaicin, or complete Freund adjuvant (CFA), is used to develop inflammatory pain models in mice.19,28,42 Injecting CFA into the hind paw of mice promotes Ser880 phosphorylation in glutamate receptor subunit 2 (GluR2) and subsequent GluR2 internalization, which leads to the increasing Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors on neuron membrane.3 More permeability allowing for more influx of Ca2+ results in the inflammatory pain hypersensitivity.
Electroacupuncture (EA) is one of the typical treatments of traditional Chinese medicine, and it is widely used in clinical settings to relieve pain.4,5,26,44 The application of acupuncture has been approved for 77 diseases by the World Health Organization since 2002.13,41 AMPA, the excitatory receptor, and its subtype (GluR2) are considered to be the key to many forms of synaptic plasticity,15,34 and EA shows a direct effect on synaptic plasticity.10,17,34,44,49 In addition, several studies12,16,46,47 suggested AMPA receptor in spinal dorsal horn (SDH) might involve EA analgesia. And, the blocking of AMPA receptors can enhance the analgesic effect of acupuncture.11,20 However, the participation of GluR2 in EA analgesia has not been well understood.21,22 They only found that the increasing GluR2-p was blocked by EA treatment.
Islet-cell autoantigen 69 (ICA69), a protein encoded by the human ICA1 or murine Ica1 gene, was initially found in patients with type I diabetes.2 Further studies showed that ICA69 is associated with autism spectrum disorder and many autoimmune diseases such as rheumatoid arthritis and primary Sjogren syndrome, all of which are associated with deficient immune tolerance of ICA69.27,30,40 ICA69 protein and its Caenorhabditis elegans homolog ric-19 are abundantly expressed in the neuroendocrine tissues, mainly in brain and pancreatic islets, and have been reported to regulate neuroendocrine secretion.6,16,29 ICA69 forms tight heteromeric complexes with protein interacting with C-kinase 1 (PICK1) through the BAR domains, and PICK1-ICA69 complexes control insulin granule trafficking.7,8 Recent studies showed that ICA69 could influence AMPA receptors, which mediate synaptic plasticity via interacting with PICK1.38,43 Our previous investigation found that absence of ICA69 enhanced the long-lasting hyperalgesia induced by subcutaneous formalin injection into the mouse hind paw. Furthermore, ICA69 knockout (KO) led to increased GluR2 phosphorylation at Ser880 in SDH.23
Given that EA could prevent the phosphorylation of GluR2, as well as the absence of ICA69 could cause GluR2 phosphorylation increasing in SDH, we hypothesized that ICA69 mediates the antihyperalgesic effects of EA by regulating GluR2 through PICK1.
2. Methods
2.1. Animals
C57BL/6 male wild-type (WT) mice, ICA69 KO, and PICK1 KO mice (10-12 weeks old) were used in this study. The original pairs of heterozygote C57BL/6 mice with a disrupted ICA69 gene (ICA69+/−) and PICK1 gene (PICK1+/−) were obtained from Ying Shen (Zhejiang University School of Medicine, Hangzhou, China) and were mated to intercross. The mice were housed at 23 ± 1°C on a standard 12:12-hours light/dark cycle, with water and food pellets available ad libitum. All experiments were approved by the Animal Experimentation Ethics Committee of Wenzhou Medical University and were specifically designed to minimize the number of animals used. To reduce the variability, the animals were trained for 1 to 2 days before being subjected to the behavior tests.
2.2. Complete Freund adjuvant–induced inflammatory pain and electroacupuncture simulation
The inflammatory pain test was performed on days −1, 0, 1, 3, 5, and 7 after CFA injection or EA according to previous work.3 In brief, 25 µL of CFA (1 mg/mL; Sigma-Aldrich, St. Louis, MO) was subcutaneously injected into the right hind paw at day 0 (CFA group). Twenty-five microliters of phosphate-buffered saline (pH 7.4) were injected as control (Sham group).
Mice in CFA+EA group received EA stimulation under anesthetization with 1% sevoflurane; Sham group and CFA group were similarly anesthetized at the same time. For the EA stimulation, 2 needles, 0.3 × 13 mm (Yunlong, China), were inserted at a depth of 1.5 mm in the right hind leg at acupoints corresponding to Zusanli (ST36) and Sanyinjiao (SP6), as described previously.22 The needles were connected to HANS LH202H Han's acupoint nerve stimulator (Beijing Hua Wei Industrial Development Co, Beijing, China). Electroacupuncture stimulation (2-15 Hz alternating wave, 1.0 mA) was initiated after CFA injection and lasted for 30 minutes. This process was repeated every 2 days (Fig. 1). Sham eletroacupuncture (SEA) was acupuncture needle insertion into acupoints without electrical stimulation or manual needle manipulation.45
Figure 1.
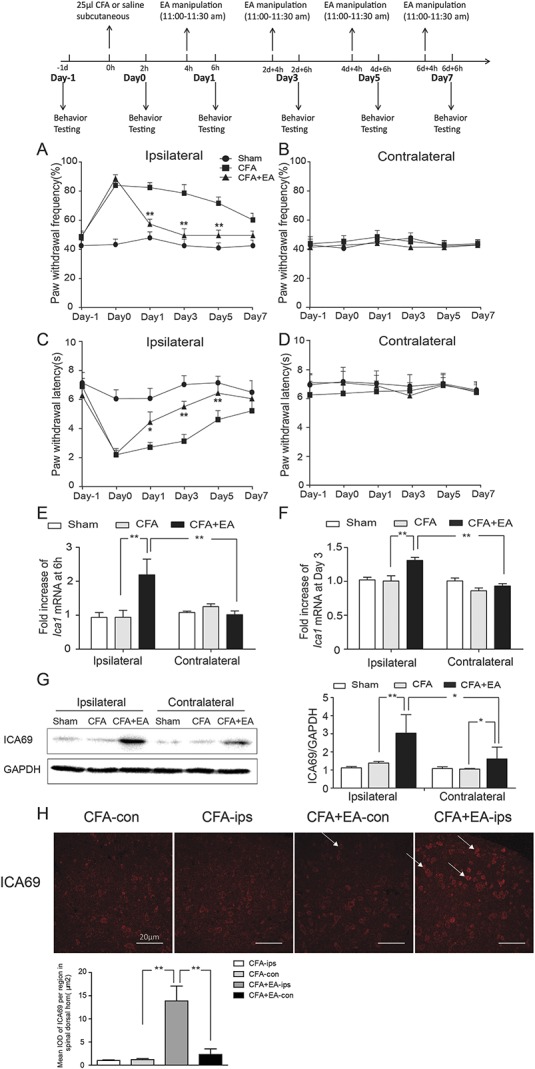
Effects of EA on CFA-induced hyperalgesia and the content of ICA69 expression. (A) Reduction in paw withdrawal frequency of the CFA+EA group compared with the CFA group on the ipsilateral side (n = 13; *P < 0.05); this reduction was particularly obvious at day 1, day 3, and day 5 (n = 13; *P < 0.05, **P < 0.01). (B) No difference among the 3 groups on the contralateral side (n = 13; P > 0.05). (C) Increase in paw withdrawal latency of the CFA+EA group compared with the CFA group on the ipsilateral side; this effect was most obvious at day 3 and day 5 (n = 13; *P < 0.05, **P < 0.01). (D) No difference among the 3 groups on the contralateral side (n = 13; P > 0.05). (E and F) Effects of EA on the expression of Ica1 mRNA in SDH at 6 hours and 3 days after CFA injection. There was an increase of the Ica1 mRNA level in the CFA+EA group compared with the CFA group on the ipsilateral side (n = 9, **P < 0.01), and an increase of the ipsilateral side compared with the contralateral side in the CFA+EA group (n = 9, **P < 0.01). (G) The ICA69 protein content increased in the CFA+EA group compared with the CFA group on the ipsilateral side (n = 9, **P < 0.01); there was an increase of the ipsilateral side compared with the contralateral side in the CFA+EA group (n = 9, *P < 0.05). (H) Immunofluorescence results of ICA69 in SDH in the 4 indicated groups: the red staining represents the ICA69 protein, and the white arrow points to the positive cells in the SDH area. Scale bars = 20 µm. Quantification of ICA69 per µm2 in SDH (n = 3; group values are indicated by mean ± SEM; **P < 0.01, *P < 0.05). CFA, complete Freund adjuvant; EA, electroacupuncture; SDH, spinal dorsal horn.
2.3. Behavioral testing
Behavioral testing was conducted as described previously.3 Briefly, paw withdrawal responses to thermal/mechanical stimuli were separately measured with a Model 336 analgesia meter (IITC Inc, Life Science Instruments, Woodland Hills, CA) and a von Frey monofilament (0.16 g; North Coast Medical Inc, Gilroy, CA). Paw withdrawal latency (PWL) (s) and paw withdrawal frequency (PWF) ([number of paw withdrawals/10 trials] × 100) were used to evaluate pain intensity.
2.4. Quantitative polymerase chain reaction
Total RNA was extracted from SDH (L3-L5) tissues of mice with TRIzol reagent (Invitrogen, Carlsbad, CA). Then, RNA was reverse transcribed into cDNAs with cDNA Synthesis kit (TaKaRa, Dalian, China). Quantitative polymerase chain reaction (qPCR) was performed with Platinum Taq DNA Polymerase (Bioline, London, United Kingdom). The primes of mouse Ica1 were as follows: forward 5′-ACCACTCTGCTCCACTTTT-3′ and reverse 5′-TTCTTCCCTTCTTTCTCAACT-3′. Mouse Gapdh was used as the internal reference gene and was amplified with the following primers: forward 5′-AAGAAGGTGGTGAAGCAG-3′ and reverse 5′-AGGTGGAAGAGTGGGAGT-3′.
2.5. Plasma membrane fraction isolation
The plasma membrane fraction was extracted as described previously.1,23 In brief, SDH (L3-L5) tissues of mice were homogenized in cold isolation buffer. The homogenate was centrifuged three times as required, and the final precipitation was the the plasma membrane fraction.
2.6. Coimmunoprecipitation and Western blot
SDH (L3-L5) tissues of mice were lysed in radio immunoprecipitation assay (plus phenylmethanesulfonyl fluoride) and centrifuged. The decimus supernatant was used as the input, and the remainder was used for coimmunoprecipitation (co-IP). Mouse anti-PICK1 antibody was precoupled to protein A-Sepharose beads on the table concentrator at room temperature (20-25°C) for 2 hours. Precleared solubilized preparations were incubated with the antibody. Proteins on the beads were extracted with 40 µL of 1X sodium dodecyl sulfate buffer and boiled for 5 minutes at 70°C.
The total protein from SDH was obtained with lysis buffer (Beyotime, Shanghai, China). Equal amounts of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, MA). After blocking the membranes with 5% bull serum albumin for 2 hours at room temperature, they were incubated with the primary antibodies anti-GAPDH (AP0063, Bioworld, Visalia, CA, USA), anti-ICA69 (a gift from Ying Shen), anti-GluR2 (MAB397; Millipore, MA), anti-PICK1 (75-040, NeuroMab, Davis, CA), anti-β-actin (AP0060; Bioworld, Visalia, CA), anti-N-cadherin (Ab76011; Abcam, Cambridge, United Kingdom), and anti-phospho-GluR2 (Ser880) (Ab52180; Abcam) at 4°C overnight. After washing the membranes, they were probed with the corresponding secondary antibodies before development using an ECL Western blotting detection system (BioRad, Hercules, CA).
2.7. Peptides
The peptides ICA69-α1 (YGRKKRRQRRRDADLDAKLELFHSI) and ICA69-α2 (YGRKKRRQRRRPYEFTTLKSLQDPM) and the leader peptide (YGRKKRRQRRR) were gifts from Zhen Wang. One and 10 mg/mL ICA69-α1/ICA69-α2 (1:1 mixed) peptides were dissolved in sterile, double-distilled water (ddH2O).
2.8. Intrathecal injection
This method has been described in detail by Hylden et al.18 In brief, the microsyringe was inserted into L4 or L5 spinous space and advanced carefully until the tail was seen flicking. Then, inject 5 µL volume of drug over 30 seconds. In addition, note that mice with motor impairment should be excluded.
2.9. Immunohistochemistry and immunofluorescence
The mouse spinal cord tissue was fixed with 4% paraformaldehyde for 24 hours, and cross-sections of the spinal cord tissue were obtained after being embedded in paraffin. Anti-GluR2-p was used to detect corresponding proteins in the sections. The sections were stained with diaminobenzidine for 15 seconds.
Donkey serum (5%) was used to block the nonspecific binding sites at room temperature for 1 hour. The sections were incubated with primary antibodies against PICK1 and ICA69 overnight at 4°C. After washing with phosphate-buffered saline thrice, the sections were incubated with the fluorescent secondary antibodies (Jackson) DyLight 488 goat anti-mouse lgG (H + L) and DyLight 594 donkey anti-rabbit lgG (H + L) for 1 hour at room temperature. Finally, the nuclei were stained with 4',6-diamidino-2-phenylindole and imaged with Leica Sp5 II laser confocal microscope.
2.10. Statistical analysis
Data analysis and original image productions were performed using Prism5 software. Statistical differences were determined using one-way analysis of variance for Western blots and qPCR, and 2-way repeated-measures analysis of variance was applied for the data from the behavioral tests. Data in the text and figures are shown as mean ± SEM. Statistical significance was accepted when P < 0.05. All statistical analyses were conducted with SPSS 19.0 software.
3. Results
3.1. Electroacupuncture significantly relieved the complete Freund adjuvant–induced hyperalgesia and increased ICA69 expression in SDH
We used the measurements of PWF and PWL to examine the extent of hyperalgesia induced by CFA injection and the antihyperalgesic effects of EA. The mice of CFA group developed mechanical and thermal hyperalgesia, which lasted for 7 days on the ipsilateral side (CFA vs Sham PWF P < 0.001, PWL P < 0.001), but not on the contralateral side (CFA vs Sham PWF P = 0.572, PWL P = 0.296). However, EA treatment at ST36 reliably inhibited the CFA-induced increase in PWF at day 1 (P < 0.001), day 3 (P < 0.001), and day 5 (P < 0.001), and blocked the CFA-induced reduction in PWL at day 1 (P = 0.022), day 3 (P = 0.001), and day 5 (P = 0.009). No differences were observed in the contralateral hind paw among Sham, CFA, and CFA+EA groups (Figs. 1A–D). These results indicated that EA could relieve the CFA-induced inflammatory pain, and the effect was most obvious at day 3.
To determine whether the effects of EA were associated with ICA69, we performed qPCR, Western blot, immunofluorescence, and immunohistochemistry analyses. Samples for qPCR were respectively collected at 6 hours and 3 days after CFA injection. There were no significant changes in the Ica1 mRNA expression in response to CFA injection compared with Sham group at either 6 hours (P = 0.993) or day 3 (P = 0.8067). However, the Ica1 mRNA level was significantly increased on the ipsilateral side of the CFA+EA group compared with the CFA group at same time points after injection (6 hours, P = 0.001; day 3, P < 0.001). This effect was not observed on the contralateral side (Figs. 1E and F). Next, we examined the total protein content of ICA69 in Sham, CFA, and CFA+EA groups by Western blot analysis. No significant difference was observed on the ipsilateral and contralateral sides of SDH between the CFA group and the Sham group. However, EA treatment significantly increased the ICA69 protein content on the ipsilateral side compared with the CFA group (P = 0.037) (Fig. 1G). It seems that EA affects ICA69 expression on both ipsilateral and contralateral sides, as WB results show that ICA69 expression of the contralateral side increased with EA treatment (P = 0.042) (Fig. 1G). This is an interesting result of the study that we will discuss later. We also used immunofluorescence and immunohistochemistry to detect the distribution of ICA69 in SDH. Consistent with the Western blot results, the ICA69 level seemed to increase on the ipsilateral side of SDH in the CFA+EA group compared with the CFA group, whereas there was no difference in ICA69 expression on the contralateral side between the 2 groups (Fig. 1H and Supplement Figure 4, available at http://links.lww.com/PAIN/A698). These results indicated that EA relieved the CFA-induced hyperalgesia accompanied by an increase in ICA69 expression.
3.2. ICA69 expression reached the peak value around the day 3 but sham electroacupuncture had no effect
To further understand the time window of the effect of EA on ICA69, we detect the variation trend of ICA69 and related protein content in the CFA+EA group during 7 days. We found that ICA69 started to rise at day 1 (P = 0.002), and the expression reached the peak value around day 3 (P < 0.001), and gradually decreased later, indicating that EA could affect the content of ICA69. (Supplement Figure 1B, available at http://links.lww.com/PAIN/A698). It is known that ICA69 could influence AMPA receptors through interacting with PICK138,43 and the increasing GluR2-p could be blocked by EA treatment. So, we also detected the variation trend of GluR2-p, GluR2, and PICK1. We found that GluR2-p started to fall off at day 1 (P < 0.001), and the expression reached the bottom value around day 3 (P < 0.001), and gradually increased later (Supplement Figure 1C, available at http://links.lww.com/PAIN/A698). And, there was no significant difference in total amount of GluR2 and PICK1 (Supplement Figures 1D and E, available at http://links.lww.com/PAIN/A698).
To ensure the ICA69 is specific in EA analgesia, we performed sham EA manipulation. The results showed that ICA69 expression was not increased in the CFA + SEA group (P = 0.386) (Supplement Figure 3B, available at http://links.lww.com/PAIN/A698), GluR2-p was not decreased (Supplement Figure 3C, available at http://links.lww.com/PAIN/A698), and there was no statistical difference in PICK1 and GluR2 compared with the CFA group (Supplement Figures 3D and E, available at http://links.lww.com/PAIN/A698), indicating that sham EA had no effect on ICA69 and GluR2-p EA was specific.
3.3. ICA69 deletion impaired the antihyperalgesic effects of electroacupuncture
Based on our results that EA increased the content of ICA69, we used a genomic strategy to directly reveal the role of ICA69 in the analgesic effects of EA with ICA69-KO mice. ICA69-KO mice show normal spontaneous motor activity compared with their WT littermates (ICA69-WT mice).23 We performed CFA injection with or without EA treatment on ICA69-KO mice and their WT littermates. There was no significant difference in PWF (P = 0.195) and PWL (P = 0.408) between the KO-Sham group and WT-Sham group, indicating that ICA69 KO did not affect the baseline of the plantar pain threshold. Injection of CFA induced a significant decrease in ICA69-KO mice (KO-Sham vs KO-CFA PWF P < 0.001, PWL P < 0.001), but no such difference was detected between WT and KO mice (KO-CFA vs WT-CFA PWF P = 0.560, PWL P = 0.818), indicating that ICA69 KO did not increase the intensity of CFA-induced hyperalgesia. However, the hyperalgesia intensity of the KO-CFA+EA group was significantly aggravated compared with the WT-CFA+EA group on the ipsilateral side (PWF P = 0.001, PWL P = 0.006), whereas no differences were seen between the KO-CFA and KO-CFA+EA groups (Figs. 2A–D). These effects were not observed on the contralateral side. This indicated that the analgesic effects produced by EA treatment were weakened in ICA69-KO mice, suggesting that ICA69 is necessary for EA analgesia.
Figure 2.
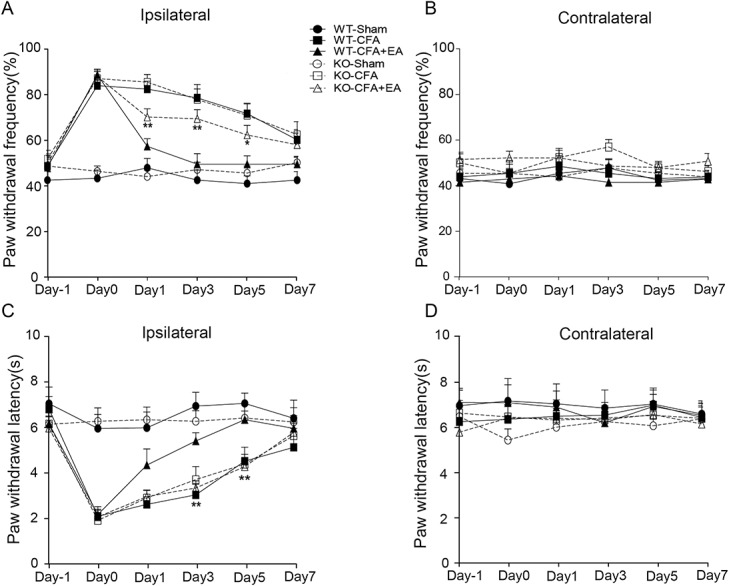
Effects of EA on CFA-induced hyperalgesia in ICA69-WT and ICA69-KO mice. (A) An increase in paw withdrawal frequency of the KO-CFA+EA group compared with the WT-CFA+EA group on the ipsilateral side (n = 13; *P < 0.05); this effect was obvious at day 1, day 3, and day 5 (n = 13; *P < 0.05, **P < 0.01). There was no difference between the KO-CFA+EA and the KO-CFA group (n = 13; P > 0.05), and there was no difference between KO-CFA and WT-CFA groups (n = 13; P > 0.05). (B) There was no difference among the 6 groups on the contralateral side (n = 13; P > 0.05). (C) Reduction in paw withdrawal latency of the KO-CFA+EA group compared with the WT-CFA+EA group on the ipsilateral side (n = 13; *P < 0.05); this effect was particularly obvious at day 3 and day 5 (n = 13; *P < 0.05, **P < 0.01). There was no difference between the KO-CFA+EA group and the KO-CFA group (n = 13; P > 0.05), and there was no difference between KO-CFA and WT-CFA groups (n = 13; P > 0.05). (D) No difference among the 6 groups was detected on the contralateral side (n = 13; P > 0.05). CFA, complete Freund adjuvant; EA, electroacupuncture; KO, knockout; WT, wild-type.
3.4. ICA69 deletion impaired the effects of electroacupuncture on the suppression of endogenous GluR2
Our previous investigation showed that the loss of ICA69 increased GluR2 phosphorylation in SDH after subcutaneous formalin injection into the mouse hind paws.23 To explore the reason why the analgesic effects of EA were weakened in the ICA69-KO mice, we examined the content of GluR2-p, which plays a pivotal role in the spinal transmission of nociceptive information in ICA69-WT and ICA69-KO mice. We confirmed that the GluR2-p level significantly increased after CFA injection compared with the Sham group in both ICA69-WT (WT-Sham vs WT-CFA, P < 0.001) and ICA69-KO mice (KO-Sham vs KO-CFA, P < 0.001). Furthermore, the GluR2-p level of the KO-CFA group was significantly increased compared with WT-CFA group (P = 0.005), which supported that ICA69 KO leads to an increase of GluR2-p.23 Electroacupuncture reduced the GluR2-p level to the baseline level in WT mice after CFA (WT-CFA vs WT-CFA+EA, P < 0.001), which was related to the analgesic effects confirmed by relevant studies. Electroacupuncture significantly downregulated GluR2-p expression in WT mice but not in ICA69-KO mice (KO-CFA+EA vs WT-CFA+EA, P < 0.001) (Figs. 3A and B).
Figure 3.
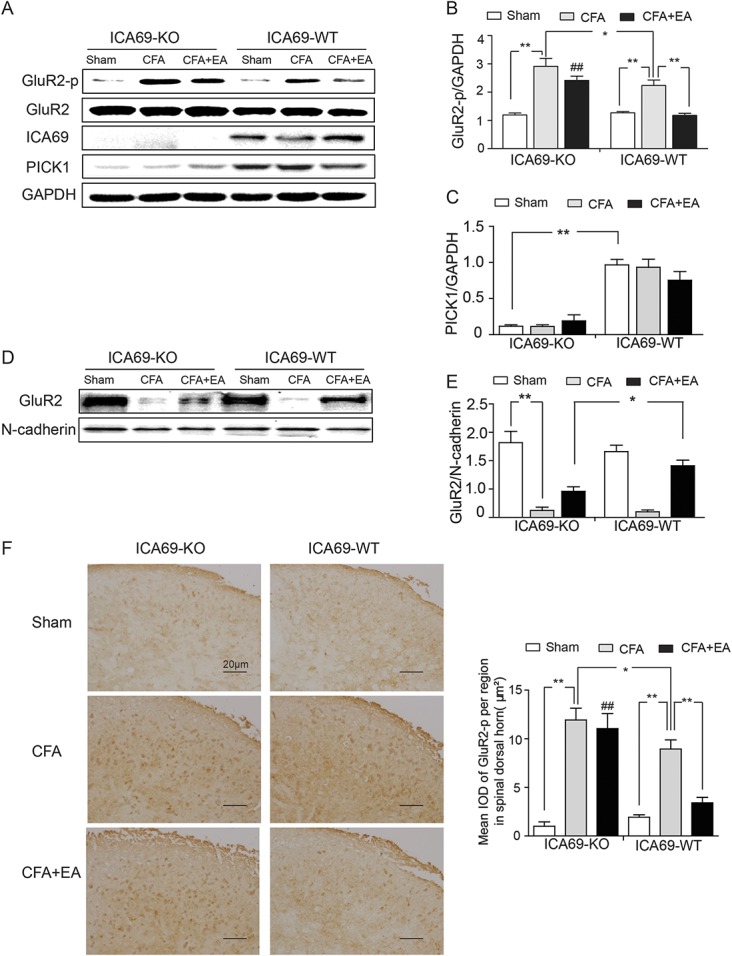
Effects of EA on the protein levels of GluR2-p, ICA69, PICK1, GluR2 (total), and GluR2 (membrane) in ICA69-WT and ICA69-KO mice. (A) Representative Western blot bands of GluR2-p, GluR2 (total), ICA69, and PICK1. Quantitative analyses of GluR2-p (##KO-CFA+EA vs WT-CFA+EA, P < 0.001) (B), PICK1 (C), and GluR2 (membrane) (E) (n = 3, group values are indicated by mean ± SEM; **P < 0.01; *P < 0.05). (D) Representative Western blot bands of GluR2 (membrane); (n = 3, group values are indicated by mean ± SEM; **P < 0.01; *P < 0.05). (F) Immunohistochemical results of GluR2-p in SDH in the 6 indicated groups: the tawny staining represents the GluR2-p protein. Scale bar = 20 μm. Quantification of GluR2-p per µm2 in SDH (n = 3; ##KO-CFA+EA vs WT-CFA+EA, P < 0.001; group values are indicated by mean ± SEM; **P < 0.01; *P < 0.05). CFA, complete Freund adjuvant; EA, electroacupuncture; IOD, integrated optical density; KO, knockout; SDH, spinal dorsal horn; WT, wild-type.
As the behavioral analysis showed that the absence of ICA69 weakened but not completely abolished the relatively reduced intensity of the antihyperalgesic effects of EA, the WB results suggested that ICA69 deficiency likely weakened the regulation of GluR2-p by EA, but did not completely block the effect. Given that there were 2 factors (ICA69 KO and EA treatment) that influenced the level of GluR2-p, a further experiment of GluR2 membrane protein isolation was performed. These results showed that the GluR2 membrane protein content of the WT-CFA+EA group was significantly higher than that in the KO-CFA+EA group (P = 0.013) (Figs. 3D and E). This also suggested that the absence of ICA69 weakened, not completely abolished, the protective effect of EA on GluR2 to the cell membrane. We also performed an immunohistochemistry experiment, and the trend was consistent with Western blot results (Fig. 3F). In addition, EA did not affect the content of PICK1 in both ICA69-WT and ICA69-KO mice (Fig. 3C). These results indicated that ICA69 KO impaired the suppression effects of EA on endogenous GluR2, which can explain why the analgesic effects of EA were weakened in ICA69-KO mice.
3.5. Injecting ICA69 peptide into the intrathecal space of ICA69-knockout mice mimicked the effects of electroacupuncture analgesic and inhibited GluR2-p
If EA exerts analgesic effects through increasing the content of ICA69, it suggests that ICA69 peptide is likely to have analgesic effects. Based on our synthesized ICA69 peptide, we detected the analgesic effects of ICA69 peptide in ICA69-KO mice. Injecting 5 µL of different concentrations of ICA69 peptide into the intrathecal space of ICA69-KO mice, we evaluated the analgesic effect of ICA69 peptide within 24 hours. There was a significant reduction in PWF of the CFA+Peptide (10 mg/mL) group compared with the CFA+LP group on the ipsilateral side at 6 and 12 hours (P = 0.005, P = 0.046) (Supplement Figure 2A, available at http://links.lww.com/PAIN/A698). Then, we detected the effects of ICA69 peptide on the protein levels of GluR2, GluR2-p, ICA69, and PICK1 at 6 hours. It could recover ICA69 expression in SDH of ICA69-KO mice by injecting 5 µL of ICA69 peptide (10 mg/mL) into the intrathecal space (P < 0.001) (Supplement Figure 2C, available at http://links.lww.com/PAIN/A698). This is consistent with the results that EA increases the expression of ICA69. Then, it increased PICK1 (P = 0.001) (Supplement Figure 2E, available at http://links.lww.com/PAIN/A698) and inhibited GluR2-p (P = 0.030) (Supplement Figure 2D, available at http://links.lww.com/PAIN/A698). This mimics the analgesic effects of EA and inhibited GluR2-p. And, there was no statistical difference in GluR2 (P = 0.448) (Supplement Figure 2F, available at http://links.lww.com/PAIN/A698). Based on the results, we concluded that ICA69-PICK1-GluR2 signaling pathway may be involved in EA analgesia.
3.6. Electroacupuncture had no effect on the total amount of PICK1 protein, and did not further relieve the complete Freund adjuvant–induced hyperalgesia and GluR2-p in PICK1-knockout mice
To explore whether PICK1 was involved in EA analgesic effect, we examined the PICK1 protein content in PICK1-WT mice and behavioral outcome of PICK1-KO mice with EA treatment. Similar to the previous results of Figure 3C, we found no difference in PICK1 total protein expression on both sides among the Sham group, the CFA group, and the CFA+EA group (Fig. 4A). Next, we performed CFA injection with or without EA treatment in PICK1-KO mice and their WT littermates. PICK1-KO mice showed normal spontaneous motor activity when compared with that of their WT littermates (PICK1-WT mice). Electroacupuncture relieved the CFA-induced hyperalgesia in PICK-WT mice. However, the PWF of the KO-CFA group was significantly decreased compared with the WT-CFA group (P < 0.001). These same test results confirm those of a previous relevant study.3 Interestingly, the PICK1 knockout reduced the PWF, which was similar to the effects of EA observed in the WT-CFA+EA group (P = 0.421). In addition, there was no significant difference in the PWF detected between the KO-CFA group and the KO-CFA+EA group (P = 0.723). These data proved that EA could not further relieve the CFA-induced hyperalgesia in PICK1-KO mice. The experiment of PWL demonstrated the same result (PWL KO-CFA vs WT-CFA P = 0.006; WT-CFA+EA vs KO-CFA P = 0.613; KO-CFA vs KO-CFA+EA P = 0.219) (Figs. 4B–E). Thus, we next examined the content of GluR2-p in PICK1-KO mice to investigate the reason that EA did not further relieve the hyperalgesia in PICK1-KO mice. Again, we confirmed that EA could inhibit the GluR2-p level, indicating that PICK1-KO leads to a decrease in the phosphorylation of GluR2 (KO-CFA vs WT-CFA, P = 0.003). Similar to the behavioral results shown in Figures 4B–E, there was no difference in the amount of GluR2-p between the KO-CFA group and the KO-CFA+EA group (P = 0.881) (Fig. 4F). This result suggested that PICK1 is likely to participate in the EA analgesic effect and that ICA69 probably regulates the phosphorylation-induced internalization of GluR2 through PICK1.
Figure 4.
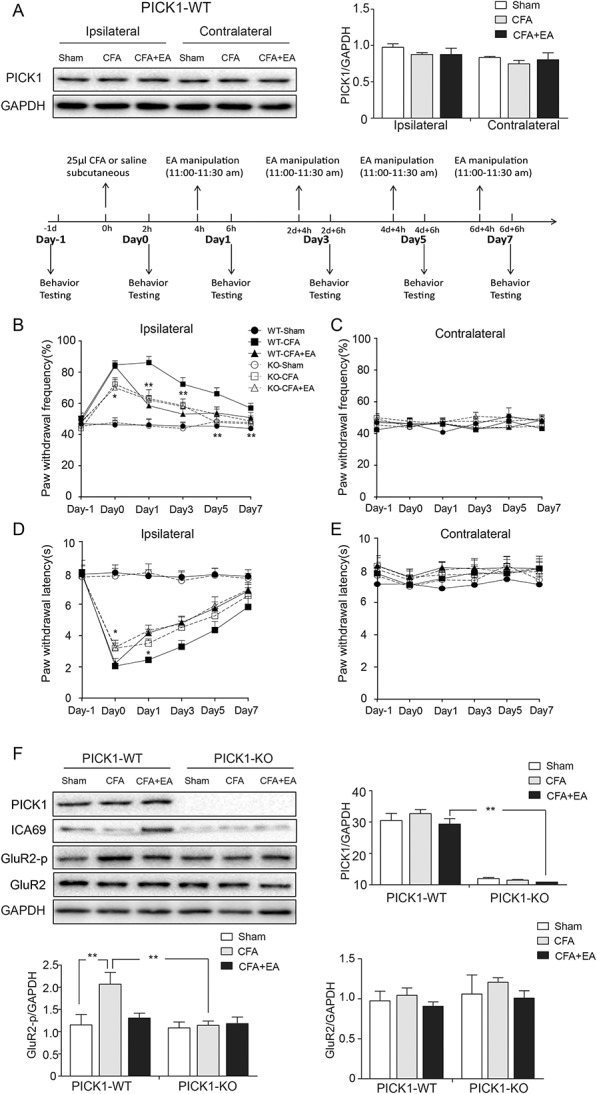
Effects of EA on CFA-induced hyperalgesia and the protein levels of PICK1, ICA69, GluR2-p, and GluR2 in PICK1-WT and PICK1-KO mice. (A) Western blot bands of PICK1 and quantitative analyses of PICK1 on both sides in PICK1-WT mice. (B) Reduction in paw withdrawal frequency of the KO-CFA group compared with the WT-CFA group on the ipsilateral side (n = 13; *P < 0.05); the effect was particularly obvious at day 0, day 1, day 3, day 5, and day 7 (n = 13; *P < 0.05, **P < 0.01). There was no difference between KO-CFA+EA and KO-CFA groups (n = 13; P > 0.05), and there was no difference between KO-CFA and WT-CFA+EA groups (n = 13; P > 0.05). (C) No difference among the 6 groups on the contralateral side (n = 13; P > 0.05). (D) Increase in paw withdrawal latency of the KO-CFA group compared with the WT-CFA group on the ipsilateral side (n = 13; *P < 0.05); the effect was particularly obvious at day 0 and day 1 (n = 13; *P < 0.05). There was no difference between KO-CFA+EA and KO-CFA groups (n = 13; P > 0.05), and there was no difference between KO-CFA and WT-CFA+EA groups (n = 13; P > 0.05). (E) There was no difference among the 6 groups on the contralateral side (n = 13; P > 0.05). (F) Representative Western blot bands of PICK1, ICA69, GluR2-p, and GluR2 in PICK1-WT and PICK1-KO mice, and quantitative analyses of these bands (n = 3, group values are indicated by mean ± SEM; **P < 0.01). CFA, complete Freund adjuvant; EA, electroacupuncture; KO, knockout; WT, wild-type.
3.7. Electroacupuncture increased the formation of ICA69-PICK1 complexes and decreased the formation of PICK1-GluR2 complexes
To explore how ICA69 mediates the EA analgesic effect through PICK1 and GluR2, we examined whether endogenous ICA69-PICK1 and PICK1-GluR2 protein complexes were regulated by EA. Using quantitative co-IP experiments, we observed a significant increase in the content of the ICA69-PICK1 complex in the CFA+EA group compared with the CFA group (P = 0.005). However, there was no significant change in the levels of ICA69-PICK1 complexes between the Sham group and the CFA group (P = 0.891) (Figs. 5A and B). This result suggested that EA could increase the rate of ICA69 binding to PICK1. To further investigate whether ICA69 regulates the internalization of GluR2 through PICK1, we also examined the content of PICK1-GluR2 complexes. Complete Freund adjuvant treatment significantly increased the content of PICK1-GluR2 (Sham vs CFA, P < 0.001), but there was a significant decrease in GluR2 binding to PICK1 in the CFA+EA group compared with the CFA group (P < 0.001) (Figs. 5A and C). On EA treatment, the ICA69 level increases, leading to an increase in the formation of ICA69-PICK1 complexes and a decrease of PICK1-GluR2 complexes.
Figure 5.
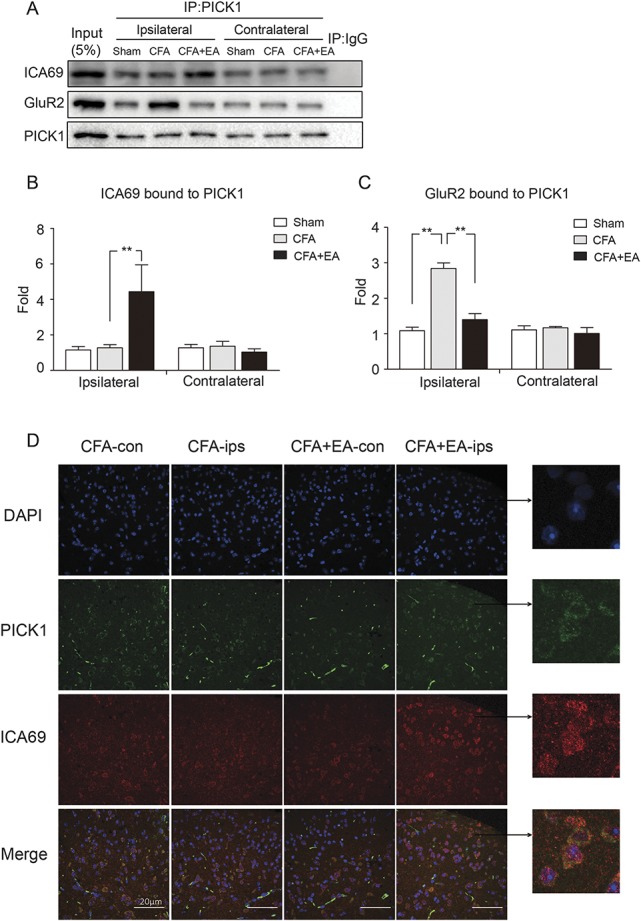
Effects of EA on the formation of endogenous ICA69-PICK1 and PICK1-GluR2 complexes. (A) Endogenous protein was collected from SDH. Representative Western blot bands of ICA69, GluR2, and PICK1 in WT mice by co-IP. (B and C) Quantitative analyses of ICA69 and GluR2 to evaluate the ability of binding to PICK1. Increased ICA69 and reduced GluR2 interactions were observed with PICK1 in the CFA+EA group (n = 3, group values are indicated by mean ± SEM; **P < 0.01). (D) Electroacupuncture caused an increase in colocalization between endogenous ICA69 (red) and PICK1 (green) on the ipsilateral side in SDH. CFA, complete Freund adjuvant; EA, electroacupuncture; SDH, spinal dorsal horn; WT, wild-type.
Next, we performed immune double staining to detect the distribution of the ICA69-PICK1 complex in SDH. Consistent with the results of quantitative co-IP, more ICA69 colocalized with PICK1 in the CFA+EA group of SDH (Fig. 5D).
4. Discussion
Increasing GluR2-p was blocked by EA treatment; however, the involvement of GluR2 in EA analgesia still remains unknown.21,22 In the current study, we demonstrate that ICA69, together with PICK1, regulates GluR2 phosphorylation, and thus mediates the antihyperalgesic effects of EA on CFA-induced inflammatory pain.
We are the first to demonstrate that EA increased the content of ICA69 in the spinal dorsal horn. ICA69 expression peaked at 3 days after EA treatment, suggesting the relationship between ICA69 expression and EA treatment. ICA69 deletion impaired the antihyperalgesic effects of EA on GluR2-p. Injecting ICA69 peptide into the intrathecal space of ICA69-KO mice mimicked the effects of EA analgesic and inhibited GluR2-p, indicating increased ICA69 expression and decreased GluR2-p expression are sufficient to EA treatment. Although EA had no effect on the total amount of PICK1 protein, EA did not further relieve the CFA-induced hyperalgesia and GluR2-p in PICK1-KO mice. This suggests that PICK1 is necessary for EA analgesia. Next, we found that EA could increase the formation of ICA69-PICK1 complexes and decrease the amount of PICK1-GluR2 complexes, suggesting that EA affected the interaction between ICA69 and PICK1, as well as GluR2 and PICK1.
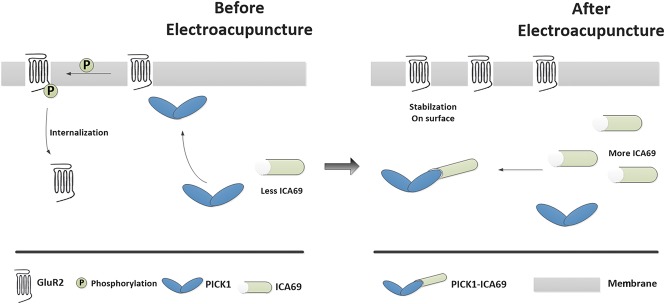
It is known that ICA69-PICK1 regulated the concentration of calcium ions through GluR2 in the nervous system, we speculated the potential mechanisms as follows (Fig. 6). On CFA injection, PICK1 is stimulated to transport around the cell membrane, leading to activation of protein kinase C-alpha to ultimately phosphorylate GluR2. Phosphorylation of GluR2 causes the internalization of GluR2 into the cytoplasm so that AMPA receptors lack the GluR2 subunit, making it permeable to Ca2+. The consequent increase in intracellular Ca2+ in SDH might initiate or potentiate a variety of Ca2+-dependent intracellular signaling cascades that are associated with the maintenance of inflammatory pain.37 On EA treatment, ICA69 increased, leading to an increase in the formation of ICA69-PICK1 complexes so that more PICK1 might be retained in the cytoplasm, which no longer induced GluR2 phosphorylation (Fig. 6). Ultimately, a series of the above-mentioned processes would not occur under EA treatment. Then, the inflammatory pain could be unsustainable. Therefore, our results suggest that ICA69 mediates the analgesic effects of EA and indicate that the trafficking of the ICA69-PICK1-GluR2 complex may be a new underlying mechanism.
Figure 6.
Proposed mechanism of the antihyperalgesic effects of EA stimulation. Before EA, there is a relatively low amount of ICA69 protein in SDH, and it does not abundantly combine with PICK1 protein in the cytoplasm; the medial PICK1 protein is therefore free to transport to the cell membrane, resulting in GluR2 phosphorylation. Once GluR2 is phosphorylated into GluR2-p, endogenous GluR2 will move from the cell membrane into the cytoplasm. AMPA receptors lacking GluR2 become permeable to Ca2+, resulting in cellular inflammation that leads to hyperalgesia. By contrast, EA treatment increases the amount of ICA69 proteins, forming abundant complexes with PICK1, thereby stranding PICK1 in the cytoplasm. This prevents GluR2 phosphorylation, and therefore AMPA receptors can maintain the impermeability of the cell membrane to Ca2+, ultimately producing an anti-inflammatory pain effect. EA, electroacupuncture.
However, how EA regulates ICA69 remains unclear. Electroacupuncture was reported to reduce the phosphorylation content of CamKII.39 Decreased CamKII phosphorylation affects the binding capacity of CamKII and PICK1.45 Given the interaction between PICK1 and ICA69, the possibility of EA affecting ICA69 exists. The specific mechanism will be a direction of our further research. Notably, the analgesic effects of EA were weakened but not completely obliterated in the absence of ICA69, due to the inhibition of GluR2 phosphorylation. Given that EA could influence multiple signaling pathways, including β-endorphins,14 5-hydroxytryptamine receptors, cannabinoid CB2 receptors,33 and adenosine A1 receptor,13 the ICA69-mediated GluR2 phosphorylation through interaction with PICK1 is likely only one of the key factors involved in EA's maintenance of analgesic effects. Moreover, PICK1 interacts with several other proteins in addition to GluR2, including acid-sensing ion channel,9 GluA1,24 CamKII,39 and dopamine receptor,48 which might also be involved in the maintenance of the analgesic effects by EA.35 Next, we will use naloxone to block opioid receptors, or DPCPX to block A1 receptors, so as to exclude the effect of other factors on EA analgesia, and then confirm the role of ICA69.
We found increase of ICA69 on the contralateral side of the CFA+EA group but there was no statistical difference (Supplement Figure 4, available at http://links.lww.com/PAIN/A698). Electroacupuncture applied at the right ST36 acupoint increased the ICA69 protein and mRNA expression levels of the ipsilateral spinal cord in mice, and the same tendency of an increase was observed in the contralateral spinal cord. The WB results (Fig. 1) showed that ICA69 expression in the contralateral side was higher in the CFA+EA group compared with the CFA group. These results were consistent with previous investigation on the effect of acupuncture or EA to the contralateral side. Song et al.32 found that EA at contralateral Zusanli (ST-36) dramatically decreased intraplantar formalin-induced increase of pERK1/2-positive neurons in the ipsilateral superficial dorsal horn of the rat. However, other scientists have shown that acupuncture did not affect the contralateral side. Goldman et al.13 found that neither 2-chloro-N(6)-cyclopentyladenosine nor acupuncture could change the pain response of the contralateral side. So, the effect of EA on the contralateral side is still controversial. The qPCR results showed that ICA69 mRNA level did not increase in the contralateral side, which may be due to decreased degradation in the contralateral side with EA. Based on previous experience, EA tends to exert overt therapeutic effects in certain pathological conditions, such as in a CFA inflammatory pain model, that are distinct from those in a normal physiological state. Therefore, further investigation is required to explore these effects and mechanisms under different conditions.
Li et al.23 show that ICA69 deficiency promoted the internalization of GluR2 and FML-induced long-lasting pain hypersensitivity. More expression of GluR2-p means more internalization of GluR2 from plasma membrane and more pain. Indeed, there was significant difference in the expression of GluR2-p between the KO-CFA group and the WT-CFA group, which is consistent to the previous study. We found that gavage of ICA69 peptides could recover ICA69 expression in the spinal dorsal horn of ICA69-KO mice. Then, it increased PICK1 and inhibited GluR2-p. ICA69 peptide (10 mg/mL) generated the analgesic effect on inflammation pain induced by CFA, which mimics the effects of EA analgesic. In addition, we found that the expression of PICK1 protein increased with the recovery of ICA69, which confirmed the interaction between PICK1 and ICA69. It indicated ICA69 as a new target for enhancing the EA analgesic efficacy, and the development and application of related drugs could be promoted furthermore.
The neuroprotective and anti-inflammation effects of EA have been verified. Because it is now established that ICA69 affects the transport of GluR2 through PICK1, this interaction could be involved in the regulation of synaptic plasticity in the central nervous system, further highlighting the theory that ICA69 mediates the neuroprotective effects of EA. Further explorations of the functions and roles of ICA69 may reveal new insight into the mechanisms underlying synaptic plasticity–associating diseases.
In conclusion, our results indicate that ICA69 mediates the antihyperalgesic effects of EA on CFA-induced inflammatory pain by regulating GluR2 phosphorylation through PICK1. Our study may provide a new target to enhance the EA analgesic efficacy.
Conflict of interest statement
The authors have no conflict of interest to declare.
Acknowledgements
The authors thank Jingjing Cheng (Department of Blood Transfusion, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College) for linguistic revision of the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (81513742, 81501824, and 81603685), Natural Science Foundation of Zhejiang Province (LY15H 290006), Wenzhou Municipal Science and Technology Bureau (Y20140706, Y20170140, Y20170214), and National 973 Project of China (2013CB531903).
Authors' contributions: HKY and MYC conceived and designed the experiments. HKY and ZAQ performed the experiments. MTT analyzed the data and drew the diagrams. QMZ and HKY wrote the paper. JBB, LD, ZXX, CSD, WZ, and WJL contributed to the paper revision, provided experimental technical support, and assisted in completing the study at different stages. All authors read and approved the final manuscript.
Ethics approval: All experimental procedures and animal care were approved by the Animal Experimentation Ethics Committee of Wenzhou Medical University (approval number wydw2014-0126) and were conducted in accordance with the guidelines of the National Institutes of Health on the care and use of animals.
Availability of data and materials: please contact author for data requests.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A698.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
K. Han, A. Zhang, and Y. Mo contributed equally to this manuscript.
Z. Wang and J. Wang are joint corresponding authors.
References
- [1].Aoyama K, Matsumura N, Watabe M, Nakaki T. Oxidative stress on EAAC1 is involved in MPTP-induced glutathione depletion and motor dysfunction. Eur J Neurosci 2008;27:20–30. [DOI] [PubMed] [Google Scholar]
- [2].Arvan P, Pietropaolo M, Ostrov D, Rhodes CJ. Islet autoantigens: structure, function, localization, and regulation. Cold Spring Harb Perspect Med 2012;2:a007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Atianjoh FE, Yaster M, Zhao X, Takamiya K, Xia J, Gauda EB, Huganir RL, Tao YX. Spinal cord protein interacting with C kinase 1 is required for the maintenance of complete Freund's adjuvant-induced inflammatory pain but not for incision-induced post-operative pain. PAIN 2010;151:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barnes PM, Barbara B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Rep 2008;12:1–23. [PubMed] [Google Scholar]
- [5].Berman BM, Lao L, Langenberg P, Lee WL, Gilpin AM, Hochberg MC. Effectiveness of acupuncture as adjunctive therapy in osteoarthritis of the knee: a randomized, controlled trial. Ann Intern Med 2004;141:901–10. [DOI] [PubMed] [Google Scholar]
- [6].Buffa L, Fuchs E, Pietropaolo M, Barr F, Solimena M. ICA69 is a novel Rab2 effector regulating ER-Golgi trafficking in insulinoma cells. Eur J Cel Biol 2008;87:197–209. [DOI] [PubMed] [Google Scholar]
- [7].Cao M, Mao Z, Kam C, Xiao N, Cao X, Shen C, Cheng KK, Xu A, Lee KM, Jiang L, Xia J. PICK1 and ICA69 control insulin granule trafficking and their deficiencies lead to impaired glucose tolerance. PLoS Biol 2013;11:e1001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cao M, Xu J, Shen C, Kam C, Huganir RL, Xia J. PICK1-ICA69 heteromeric BAR domain complex regulates synaptic targeting and surface expression of AMPA receptors. J Neurosci 2007;27:12945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen WH, Hsieh CL, Huang CP, Lin TJ, Tzen JT, Ho TY, Lin YW. Acid-sensing ion channel 3 mediates peripheral anti-hyperalgesia effects of acupuncture in mice inflammatory pain. J Biomed Sci 2011;18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chiechio S, Nicoletti F. Metabotropic glutamate receptors and the control of chronic pain. Curr Opin Pharmacol 2012;12:28–34. [DOI] [PubMed] [Google Scholar]
- [11].Choi BT, Kang J, Jo UB. Effects of electroacupuncture with different frequencies on spinal ionotropic glutamate receptor expression in complete Freund's adjuvant-injected rat. Acta Histochem 2005;107:67–76. [DOI] [PubMed] [Google Scholar]
- [12].Choi BT, Lee JH, Wan Y, Han JS. Involvement of ionotropic glutamate receptors in low frequency electroacupuncture analgesia in rats. Neurosci Lett 2005;377:185–8. [DOI] [PubMed] [Google Scholar]
- [13].Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, Chen JF, Schnermann J, Takano T, Bekar L, Tieu K, Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci 2010;13:883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci 2003;26:17–22. [DOI] [PubMed] [Google Scholar]
- [15].Hartmann B, Ahmadi S, Heppenstall PA, Lewin GR, Schott C, Borchardt T, Seeburg PH, Zeilhofer HU, Sprengel R, Kuner R. The AMPA receptor subunits GluR-A and GluR-B reciprocally modulate spinal synaptic plasticity and inflammatory pain. Neuron 2004;44:637–50. [DOI] [PubMed] [Google Scholar]
- [16].He J, Xia M, Tsang WH, Chow KL, Xia J. ICA1L forms BAR-domain complexes with PICK1 and is crucial for acrosome formation in spermiogenesis. J Cel Sci 2015;128:3822–36. [DOI] [PubMed] [Google Scholar]
- [17].Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron 2013;80:704–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol 1980;67:313–6. [DOI] [PubMed] [Google Scholar]
- [19].Iwaszkiewicz KS, Hua S. Development of an effective topical liposomal formulation for localized analgesia and anti-inflammatory actions in the complete Freund's adjuvant rodent model of acute inflammatory pain. Pain Physician 2014;17:E719–35. [PubMed] [Google Scholar]
- [20].Jang JY, Kim HN, Koo ST, Shin HK, Choe ES, Choi BT. Synergistic antinociceptive effects of N-methyl-D-aspartate receptor antagonist and electroacupuncture in the complete Freund's adjuvant-induced pain model. Int J Mol Med 2011;28:669–75. [DOI] [PubMed] [Google Scholar]
- [21].Kim YR, Kim HN, Jang JY, Park C, Choi YH, Shin HK, Choi BT. Electroacupuncture confers beneficial effects through ionotropic glutamate receptors involving phosphatidylinositol-3 kinase/Akt signaling pathway in focal cerebral ischemia in rats. Eur J Integr Med 2012;4:e413–20. [Google Scholar]
- [22].Lee YS, Lee JH, Lee IS, Choi BT. Effects of electroacupuncture on spinal alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor in rats injected with complete Freund's adjuvant. Mol Med Rep 2013;8:1130–4. [DOI] [PubMed] [Google Scholar]
- [23].Li QJ, Wang Z, Yao YX, Jin SH, Qian MZ, Li NN, Wang YN, Zhang YW, Chen BY, Jia DY, Shen Y, Wang JL. Loss of ICA69 potentiates long-lasting hyperalgesia after subcutaneous formalin injection into the mouse hindpaw. Neurochem Res 2015;40:579–90. [DOI] [PubMed] [Google Scholar]
- [24].Li YH, Zhang N, Wang YN, Shen Y, Wang Y. Multiple faces of protein interacting with C kinase 1 (PICK1): structure, function, and diseases. Neurochem Int 2016;98:115–21. [DOI] [PubMed] [Google Scholar]
- [25].Liu YN, Yang X, Suo ZW, Xu YM, Hu XD. Fyn kinase-regulated NMDA receptor- and AMPA receptor-dependent pain sensitization in spinal dorsal horn of mice. Eur J Pain 2014;18:1120–8. [DOI] [PubMed] [Google Scholar]
- [26].Lu KW, Hsu CK, Hsieh CL, Yang J, Lin YW. Probing the effects and mechanisms of electroacupuncture at ipsilateral or contralateral ST36-ST37 acupoints on CFA-induced inflammatory pain. Sci Rep 2016;6:22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Martin S, Kardorf J, Schulte B, Lampeter EF, Gries FA, Melchers I, Wagner R, Bertrams J, Roep BO, Pfutzner A. Autoantibodies to the islet antigen ICA69 occur in IDDM and in rheumatoid arthritis. Diabetologia 1995;38:351–5. [DOI] [PubMed] [Google Scholar]
- [28].Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci 2009;10:283–94. [DOI] [PubMed] [Google Scholar]
- [29].Pilon M, Peng XR, Spence AM, Plasterk RH, Dosch HM. The diabetes autoantigen ICA69 and its Caenorhabditis elegans homologue, ric-19, are conserved regulators of neuroendocrine secretion. Mol Biol Cel 2000;11:3277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Salyakina D, Cukier HN, Lee JM, Sacharow S, Nations LD, Ma D, Jaworski JM, Konidari I, Whitehead PL, Wright HH, Abramson RK, Williams SM, Menon R, Haines JL, Gilbert JR, Cuccaro ML, Pericak-Vance MA. Copy number variants in extended autism spectrum disorder families reveal candidates potentially involved in autism risk. PLoS One 2011;6:e26049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Singhmar P, Huo X, Eijkelkamp N, Berciano SR, Baameur F, Mei FC, Zhu Y, Cheng X, Hawke D, Mayor F, Murga C, Heijnen CJ, Kavelaars A. Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proc Natl Acad Sci U S A 2016;113:201516036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Song L, Zhu ZH, Duan XL, Liu XJ, Fan J, Ju G. Effects of electroacupuncture at “zusanli” (st 36) on erk1/2 phosphorylation in the dorsal horn of spinal cord of the rat. Zhongguo Zhen Jiu 2006;26:362–6. [PubMed] [Google Scholar]
- [33].Su TF, Zhang LH, Peng M, Wu CH, Pan W, Tian B, Shi J, Pan HL, Li M. Cannabinoid CB2 receptors contribute to upregulation of beta-endorphin in inflamed skin tissues by electroacupuncture. Mol Pain 2011;7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tao YX. Dorsal horn alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking in inflammatory pain. Anesthesiology 2010;112:1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Torres-Rosas R, Yehia G, Pena G, Mishra P, del Rocio Thompson-Bonilla M, Moreon-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 2014;20:291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vaughn AH, Gold MS. Ionic mechanisms underlying inflammatory mediator-induced sensitization of dural afferents. J Neurosci 2010;30:7878–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang W, Petralia RS, Takamiya K, Xia J, Li YQ, Huganir RL, Tao YX, Yaster M. Preserved acute pain and impaired neuropathic pain in mice lacking protein interacting with C Kinase 1. Mol Pain 2011;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang Z, Wang YN, Sun CL, Yang D, Su LD, Xie YJ, Zhou L, Wang Y, Shen Y. C-terminal domain of ICA69 interacts with PICK1 and acts on trafficking of PICK1-PKCalpha complex and cerebellar plasticity. PLoS One 2013;8:e83862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wei L, Khatri L, Ziff EB. Trafficking of AMPA receptor subunit GluA2 from the endoplasmic reticulum is stimulated by a complex containing CaMKII and PICK1 and by release of Ca2+ from internal stores. J Biol Chem 2014;289:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Winer S, Astsaturov I, Cheung R, Tsui H, Song A, Gaedigk R, Winer D, Sampson A, McKerlie C, Bookman A, Dosch HM. Primary Sjögren's syndrome and deficiency of ICA69. Lancet 2002;360:1063–9. [DOI] [PubMed] [Google Scholar]
- [41].World Health Organization. Acupuncture: review and analysis of reports on controlled clinical trials. Geneva: WHO, 2002. [Google Scholar]
- [42].Xiao X, Zhao XT, Xu LC, Yue LP, Liu FY, Cai J, Liao FF, Kong JG, Xing GG, Yi M, Wan Y. Shp-1 dephosphorylates TRPV1 in dorsal root ganglion neurons and alleviates CFA-induced inflammatory pain in rats. PAIN 2015;156:597–608. [DOI] [PubMed] [Google Scholar]
- [43].Xu J, Kam C, Luo JH, Xia J. PICK1 mediates synaptic recruitment of AMPA receptors at neurexin-induced postsynaptic sites. J Neurosci 2014;34:15415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture–electroacupuncture on persistent pain. Anesthesiology 2014;120:482–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang Y, Li A, Xin J, Ren K, Berman BM, Lao L, Zhang RX. Electroacupuncture alleviates chemotherapy-induced pain through inhibiting phosphorylation of spinal CaMKII in rats. Eur J Pain 2017;22:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang YQ, Ji GC, Wu GC, Zhao ZQ. Excitatory amino acid receptor antagonists and electroacupuncture synergetically inhibit carrageenan-induced behavioral hyperalgesia and spinal fos expression in rats. PAIN 2002;99:525–35. [DOI] [PubMed] [Google Scholar]
- [47].Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol 2008;85:355–75. [DOI] [PubMed] [Google Scholar]
- [48].Zheng M, Zhang X, Min C, Choi BG, Oh IJ, Kim KM. Functional regulation of dopamine D(3) receptor through interaction with PICK1. Biomol Ther 2016;24:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhou W, Longhurst JC. Neuroendocrine mechanisms of acupuncture in the treatment of hypertension. Evid Based Complement Alternat Med 2012;2012:878673. [DOI] [PMC free article] [PubMed] [Google Scholar]