Abstract
Background
Serotonin 5-HT2A and metabotropic glutamate 2 (mGlu2) are neurotransmitter G protein-coupled receptors (GPCRs) involved in the signaling mechanisms underlying psychosis and schizophrenia treatment. Previous findings in mGlu2 knockout (KO) mice suggested that mGlu2 is necessary for head-twitch behavior, a rodent phenotype characteristic of hallucinogenic 5-HT2A receptor agonists. However, the role of mGlu2 in the behavioral effects induced by antipsychotic drugs remains poorly understood. Here we tested antipsychotic-like behavioral phenotypes induced by the atypical antipsychotic clozapine in mGlu2-KO mice and wild-type control littermates.
Methods
Locomotor activity was tested in mGlu2-KO mice and control littermates injected (i.p.) with clozapine (1.5 mg/kg) or vehicle followed by MK801 (0.5 mg/kg), PCP (7.5 mg/kg), amphetamine (6 mg/kg), scopolamine (2 mg/kg), or vehicle. Using a virally (HSV) mediated transgene expression approach, the role of frontal cortex mGlu2 in the modulation of MK801-induced locomotor activity by clozapine treatment was also evaluated.
Results
The effect of clozapine on hyperlocomotor activity induced by the dissociative drugs MK801 and phencyclidine (PCP) was decreased in mGlu2-KO mice as compared to controls. Clozapine treatment, however, reduced hyperlocomotor activity induced by the stimulant drug amphetamine, and the deliriant drug scopolamine in both wild-type and mGlu2-KO mice. Virally mediated over-expression of mGlu2 in the frontal cortex of mGlu2-KO mice rescued the ability of clozapine to reduce MK801-induced hyperlocomotion.
Conclusion
These findings further support the existence of a functionally relevant crosstalk between 5-HT2A and mGlu2 receptors in different preclinical models of antipsychotic activity.
Keywords: Schizophrenia, antipsychotics, hallucinogens, GPCR complexes, clozapine, LSD, serotonin, 5-HT2A receptor, mGlu2 receptor
Introduction
Schizophrenia, as currently defined, represents a unique human disorder, and modeling schizophrenia-related phenotypes in rodents remains challenging (Arguello and Gogos 2006; Fernando and Robbins 2011; Jones et al. 2011). Despite displaying very distinct pharmacological profiles, stimulant drugs such phencyclidine (PCP) and MK801 (noncompetitive NMDA receptor antagonists) (Malhotra et al. 1997; Umbricht et al. 2000; Umbricht et al. 2002), amphetamine (which, among other mechanisms, derives its psychoactive effects through augmentation of dopamine efflux via the dopamine transporter) (Featherstone et al. 2007; Bramness et al. 2012), and scopolamine (nonspecific muscarinic receptor antagonist) (Young et al. 2013; Ham et al. 2017), induce behavioral disruptions in healthy subjects that present certain similarities with those observed in schizophrenia patients (Steeds et al. 2015). Some of these disruptive behaviors and their prevention with antipsychotics have been successfully reproduced in animal models. For instance, modulation of hyperlocomotor activity induced by MK801 has already been validated as an assay for detection of antipsychotic action (Bradford et al. 2010).
Despite bearing a complex polypharmacology (Meltzer 2013), clozapine and other atypical antipsychotics, such as olanzapine and risperidone, possess a very high affinity for the serotonin 5-HT2A receptor, alongside with a lower affinity for the dopamine D2 receptor (Lieberman et al. 2008; Miyamoto et al. 2012). Based on assays in heterologous expression systems such as NIH-3T3 and COS-7 cells, it was postulated that atypical antipsychotics share a pharmacological property: inverse agonism for the 5-HT2A receptor (Sullivan et al. 2015). More recent findings suggest that clozapine also behaves as a partial agonist activating certain signaling pathways downstream of the 5HT2A receptor, such as Akt phosphorylation (Schmid et al. 2014). Long-lasting exposure to clozapine induces 5-HT2A receptor internalization in tissue culture (Bhatnagar et al. 2001; Raote et al. 2013), and down-regulation of frontal cortex 5-HT2A receptor density in rodent models (Yadav et al. 2011; Moreno et al. 2013; Ibi et al. 2017). These processes of 5-HT2A receptor internalization and down-regulation after chronic exposure to clozapine treatment have been postulated to be one of the possible mechanisms underlying clozapine’s antipsychotic action. Nevertheless, in schizophrenia patients many clinical studies show improvement hours/days immediately after antipsychotic drug administration (Agid et al. 2003). This underscores the need for further research into the basic mechanisms underlying acute therapeutic effects upon antipsychotic drug administration.
The serotonin 5-HT2A receptor is responsible for most of the signaling and psychoactive effects induced by hallucinogenic drugs (also termed psychedelics), such as lysergic acid diethylamide (LSD), psilocin and mescaline (Nichols 2004; 2016). Rodent behavior models of hallucinogenic drug action, such as head-twitch behavior (Hanks and Gonzalez-Maeso 2013), are blocked by 5-HT2A receptor antagonists (Willins and Meltzer 1997; Fantegrossi et al. 2010). This hallucinogen-dependent behavioral phenotype is also abolished in 5-HT2A knockout (KO) mice (Gonzalez-Maeso et al. 2003; Gonzalez-Maeso et al. 2007). Similar findings supporting a role for 5-HT2A receptor-dependent signaling processes in the mechanism of action of hallucinogens have been reported in humans. Thus, the psychedelic effects of LSD and psilocin are blocked by the 5-HT2A receptor antagonist ketanserin in healthy volunteers (Vollenweider et al. 1998; Schmid et al. 2015; Preller et al. 2017). Our previous findings suggested that the 5-HT2A receptor-dependent head-twitch behavior induced by the hallucinogens DOI and LSD is significantly reduced in mGlu2-KO mice as compared to wild-type littermates (Moreno et al. 2011) – this notion has recently been supported by the demonstration that administration of the hallucinogenic 5-HT2A receptor agonist DOI elicited a dose-dependent head-twitch behavioral effect in wild-type mice, a phenotype that was abolished in mGlu2-KO, but not in mGlu3-KO, mice (Benvenga et al. 2018).
Additionally, our previous findings demonstrated that the ability of clozapine to prevent MK801-induced hyperlocomotor activity was significantly decreased in mGlu2-KO mice (Fribourg et al. 2011). These results suggested that the mGlu2 receptor is necessary to induce certain 5-HT2A receptor-dependent antipsychotic-related behavioral phenotypes. However, it remains unsolved whether the mGlu2 receptor plays a role in the psychosis-like behaviors induced by PCP, amphetamine and scopolamine in rodents, or in the blockade of these phenotypes by antipsychotics.
G protein-coupled receptors (GPCRs) were assumed to exist and function as monomeric structural units. However, more recent findings suggest that GPCRs may be assembled as homomeric and/or heteromeric protein complexes under certain experimental conditions (Gonzalez-Maeso 2011; 2014). We and others have shown a laminar co-distribution of 5-HT2A and mGlu2 in layers II/II and V of the frontal cortex that is not apparent in other cortical and subcortical regions (Marek et al. 2000; Gonzalez-Maeso et al. 2008). Additionally, we previously reported that 5-HT2A and mGlu2 receptors form a highly specific GPCR heteromeric complex in living mammalian cells (Gonzalez-Maeso et al. 2008; Fribourg et al. 2011; Moreno et al. 2012; Baki et al. 2016; Moreno et al. 2016).
In the present study, we tested the role of the mGlu2 receptor in repression of hyperlocomotor activity induced by a battery of psychoactive drugs upon clozapine administration. Using a virally (HSV) mediated over-expression approach, we also tested whether frontal cortex mGlu2 expression is sufficient to modulate the effect of clozapine treatment on MK801-induced hyperlocomotor activity. This was performed in parallel with HSV-mediated over-expression of an mGlu2/mGlu3 chimeric construct that, based on our previous findings in heterologous expression systems (Moreno et al. 2012), is unable to form a GPCR heteromeric complex with the 5-HT2A receptor.
Methods
Animals
Experiments were performed on adult (10–20 weeks old) male mice. Animals were housed at 12 h light/dark cycle at 23°C with food and water ad libitum, except during behavioral testing. Experiments were conducted in accordance with NIH guidelines, and were approved by the Virginia Commonwealth University Animal Care and Use Committee. All efforts were made to minimize animal suffering and the number of animals used.
mGlu2 knockout (KO) mice have been described previously (Yokoi et al. 1996; Moreno et al. 2011). For experiments involving mGlu2-KO mice, wild-type littermates in the 129S6/Sv background were used as controls. All subjects were offspring of heterozygote breeding.
Behavioral testing took place between 9:00 a.m. and 6:00 p.m. Each behavioral paradigm was separated at least one week for a wash-out period.
Materials and drug administration
1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane (DOI), (5R,10S)-(+)-5-methyl-10,11dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (dizocilpine, (+)MK801), 1-(1-phenylcyclohexyl)-piperidine (phencyclidine, PCP), 1-phenylpropan-2amine (amphetamine), and scopolamine were purchased from Sigma-Aldrich. Clozapine was obtained from Tocris. DOI, MK801, PCP and scopolamine were injected (i.p.) after suspension in a minimal amount of DMSO and made up to volume with normal saline. Clozapine was injected (i.p.) after dissolution in a minimal volume of DMSO supplemented with acetic acid and made up to volume with normal saline. The injected doses were as follows: DOI, 1 mg/kg; clozapine, 1.5 mg/kg; MK801, 0.5 mg/kg; PCP, 7.5 mg/kg; amphetamine, 6.0 mg/kg and scopolamine, 2 mg/kg. Doses of DOI, and MK801 were selected based on previous studies (Gonzalez-Maeso et al. 2007; Fribourg et al. 2011; Moreno et al. 2011; Kurita et al. 2012; Ibi et al. 2017). Doses of PCP, amphetamine and scopolamine were selected based on previous studies (Fell et al. 2008; Woolley et al. 2008; Lavreysen et al. 2015), and on pilot dose-response assays (data not shown). The dose of clozapine (1.5 mg/kg) was selected based on our previous findings with dose-response assays with which we demonstrated that the effect of clozapine (1.5 mg/kg) on MK801-induced hyperlocomotor activity was absent in 5-HT2A-KO mice (Fribourg et al. 2011), whereas the effect of higher doses of clozapine on MK801-induced hyperlocomotor activity were observed in both wild-type and 5-HT2AKO mice (Fribourg et al. 2011). Vehicle-treated mice received 0.9% saline (0.1 ml/g body weight).
Locomotor behavior
Motor function (exploratory behavior) was assessed using a computerized threedimensional activity monitoring system (Omnitech). The system determines motor activity based on frequency of interruptions to infrared beams traversing the x, y and z planes. For basal locomotor activity, mice were monitored for 90 min (dimensions of the arena: 27 × 27 × 21 cm). For center time, the proportion of time spent in the center of the arena was interpreted as a measure of anxiety (dimensions of the arena: 41 × 41 × 30 cm). For modulation of locomotor activity post-drug administration, mice first were left to habituate in the locomotor box (dimensions of the arena: 27 × 27 × 21 cm) for 90 min, during which mice were able to explore the test chamber to mitigate noveltyinduced effects. After habituation, mice were injected (i.p.) with clozapine, or vehicle. A 5-min lag in the test chamber followed (not shown in the analysis), after which MK801, PCP, amphetamine or scopolamine were administered (i.p.). Mice were monitored for an additional 120-min period during the test phase. Locomotor activity was automatically determined from the interruptions of beams in the horizontal and vertical planes. All locomotor behavior experiments were carried out at 16 lux. Time blocks to test locomotor activity upon administration of psychoactive drugs (MK801, PCP and amphetamine: 20–80 min and 85–120 min; and scopolamine: 5–80 min and 85–120 min) were selected based on pilot assays that evaluated the temporal phases in which clozapine (1.5 mg/kg) affects hyperlocomotor activity (data not shown).
Virally mediated over-expression
The constructs mGlu2 and mGlu2ΔTM4N, in which the intracellular end of TM4 of mGlu2 is replaced with the intracellular end of TM4 of mGlu3, sub-cloned into a bicistronic HSV-GFP vector have been described before (Moreno et al. 2012). HSVmGlu2, HSV-mGlu2ΔTM4N, or control HSV-GFP was injected into the frontal cortex by stereotaxic surgery according to standard methods (Kurita et al. 2012; Moreno et al. 2012; Ibi et al. 2017). Mice were anesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg) during the surgery. The virus was delivered bilaterally with a Hamilton syringe at a rate of 0.1 μl/min for a total volume of 0.5 μl on each side. The following coordinates were used: +1.6 mm rostral-caudal, −2.4 mm dorsal-ventral, +2.6 mm medial-lateral from bregma (relative to dura) with a 10° lateral angle. The coordinates were taken according to a published atlas of the 129/Sv mouse strain (Hof et al. 2000). The Nissl staining patterns of the coronal brain slice were taken from the mouse brain atlas with author’s permission. All experiments were performed 3–4 d after the viral infection, when transgene expression is maximal. Virally mediated mGlu2 and mGlu2ΔTM4N over-expression levels in frontal cortex have previously been confirmed by Western blotting (Moreno et al. 2012).
Immunohistochemistry
Immunohistochemistry assays were performed as previously reported with minor changes (Kurita et al. 2012; Moreno et al. 2012). The animals were deeply anesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg) prior to the surgery. Transcardiac perfusion was performed with 10 ml PBS, followed by 30 ml of freshly prepared 4% paraformaldehyde (PFA) in PBS at room temperature. Brains were removed and immersion-fixed in 4% PFA in PBS at 4°C (overnight), and stored at 30% sucrose in PBS for at least 48 h at 4°C. Screening of immunoreactive cells used a series of 20 μm-thick coronal sections from frontal cortex prepared on a sliding vibratome (Leica VT1000S). The free-floating sections were transferred to 24-well dishes containing PBS. Coronal brain sections were washed with PBS and incubated in 5% bovine serum albumin with 0.1% Triton X-100 in PBS for 60 min at 4°C. The sections were then incubated overnight in the same solution containing the anti-GFP antibody (Invitrogen A11122; 1:1000). The sections were rinsed 5 times in PBS for 10 min and incubated for 1 h with Alexa 488 dye-conjugated goat anti-rabbit antibody (1:2000). Following incubation, the sections were washed three times with PBS, and the immunostained sections were examined by epifluorescence microscopy (AxioImager Z1, Carl Zeiss).
Statistical analysis
Statistical analyses were performed with GraphPad Prism software version 6. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in our previous publications. Data distribution was assumed to be normal but this was not formally tested. Animals were randomly allocated into the different experimental groups. Statistical significance of experiments involving three or more groups and two or more treatments was assessed by two-way ANOVA followed by Bonferroni’s post hoc test. Statistical significance of experiments involving three or more groups was assessed by one-way ANOVA followed by Bonferroni’s post hoc test. Statistical significance of experiments involving two groups was assessed by Student’s t-test. The level of significance was chosen at p = 0.05. All values included in the figure legends represent mean ± SEM.
Results
Exploratory behavior
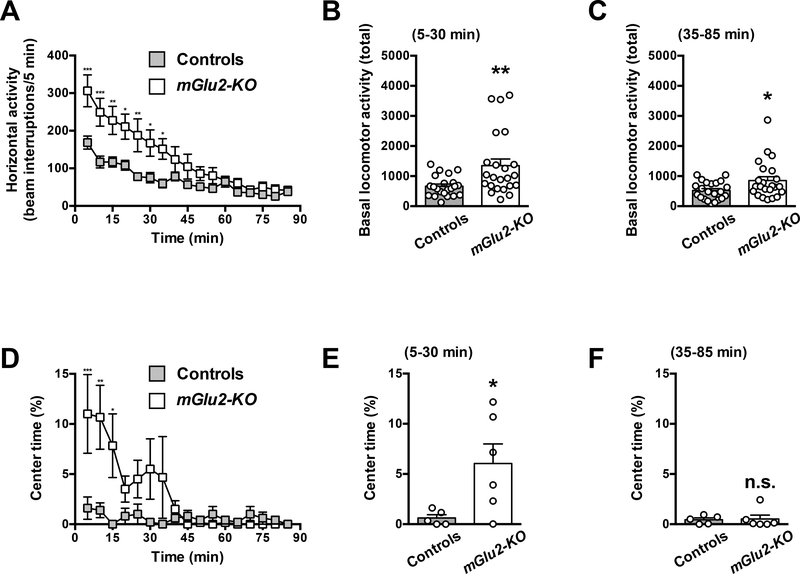
Two-way ANOVA revealed that there was a significant effect of genotype on spontaneous locomotor activity (exploratory behavior) (Fig. 1A) (F1,43 = 7.60; P < 0.01). Summation of horizontal activity from t = 5 to t = 30 min was increased in mGlu2-KO mice as compared to wild-type littermates (Fig. 1B) (t43 = 2.91; P < 0.01). Summation of horizontal activity from t = 35 to t = 85 min was also increased in mGlu2-KO mice as compared to wild-type littermates (Fig. 1C) (t43 = 2.26; P < 0.05).
Fig. 1.
Exploratory activity in the open field test. (A-F) Wild-type and mGlu2-KO mice were placed in the locomotor chamber, and allowed to habituate for 5 min. After habituation, horizontal activity (n = 22–23) and time spent in the center of the arena (n = 5–6) was measured for 85 min. Horizontal activity (A-C) and the proportion of time spent in the center of the arena (D-F) are shown. Time courses (A,D) and total counts from t = 5 to t = 30 min (B,E) and from t = 35 to t = 85 min (C,F) are shown for each measurement. *P < 0.05, **P < 0.01, ***P < 0.001, Bonferroni’s post hoc test of two-way ANOVA (A,D) or unpaired Student’s t-test (B,C,E,F). Data are means ± SEM, n.s., not significant.
Two-way ANOVA revealed that there was trend towards an effect of genotype on center time (Fig. 1D) (F1,9 = 3.69; P = 0.08). The trend for augmented center time from t = 5 to t = 30 min was also evident in mGlu2-KO mice as compared to wild-type littermates (Fig. 1E) (t9 = 2.47; P < 0.05). Summation of horizontal activity from t = 35 to t = 85 min was unaffected in mGlu2-KO mice as compared to wild-type littermates (Fig. 1F) (t9 = 0.15; P > 0.05).
Effect of the hallucinogen DOI on locomotor activity
Two-way ANOVA revealed that there was a significant effect of DOI treatment on locomotor activity time course from t = 20 to t = 120 min in wild-type mice (Fig. 2A) (F1,16 = 11.16; P < 0.01). Two-way ANOVA revealed that there was not a significant effect of DOI treatment on locomotor activity time course from t = 20 to t = 120 min in mGlu2-KO mice (Fig. 2B) (F1,16 = 0.23; P > 0.05).
Fig. 2.
Effect of DOI on locomotor behavior. (A,D) Wild-type and mGlu2-KO mice were placed in the locomotor chamber, and allowed to habituate for 90 min. After habituation, mice were injected with DOI (1.0 mg/kg) or vehicle and locomotor activity was measured for another 120 min (n = 6–12). (A,B) The panel depicts the time course of DOI-induced locomotion in 5 min blocks. Time of injection is indicated by arrow. (C,D) Data summary of the total DOI-induced locomotion as a summation of horizontal activity from t = 20 to t = 80 min (C) and from t = 85 to t = 120 min (D). *P < 0.05, **P < 0.01, ***P < 0.001, Bonferroni’s post hoc test of two-way ANOVA. Data are means ± SEM, n.s., not significant.
Two-way ANOVA also indicated that there was not a significant effect of DOI treatment on summation of locomotor activity from t = 20 to t = 80 min (Fig. 2C) (F1,32 = 2.18; P > 0.05). Two-way ANOVA revealed that there was a significant effect of genotype on summation of locomotor activity from t = 20 to t = 80 min (Fig. 2C) (F1,32 = 6.74; P < 0.05). Post hoc analysis indicated that DOI increased locomotor activity from t = 20 to t = 80 min in wild-type mice (P < 0.05), but not in mGlu2-KO mice (Fig. 2C) (P > 0.05). Post hoc analysis also showed absence of differences in locomotor activity from t = 20 to t = 80 min between vehicle-treated wild-type mice and vehicle-treated mGlu2-KO mice (Fig. 2C) (P > 0.05).
Two-way ANOVA indicated that there was a significant effect of DOI treatment on summation of locomotor activity from t = 85 to t = 120 min (Fig. 2D) (F1,32 = 4.22; P < 0.05). Two-way ANOVA revealed that there was not a significant effect of genotype on summation of locomotor activity from t = 85 to t = 120 min (Fig. 2D) (F1,32 = 0.21; P > 0.05). Post hoc analysis indicated that DOI increased locomotor activity in wild-type mice (P < 0.01), but not in mGlu2-KO mice (Fig. 2D) (P > 0.05). Post hoc analysis also showed absence of differences in locomotor activity from t = 85 to t = 120 min between vehicle-treated wild-type mice and vehicle-treated mGlu2-KO mice (Fig. 2D) (P > 0.05).
Effect of the clozapine on locomotor activity
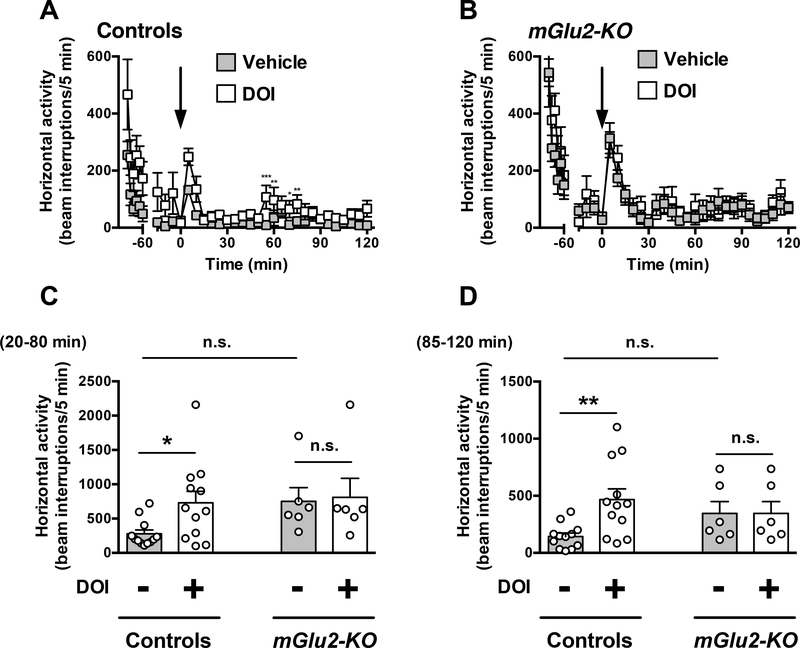
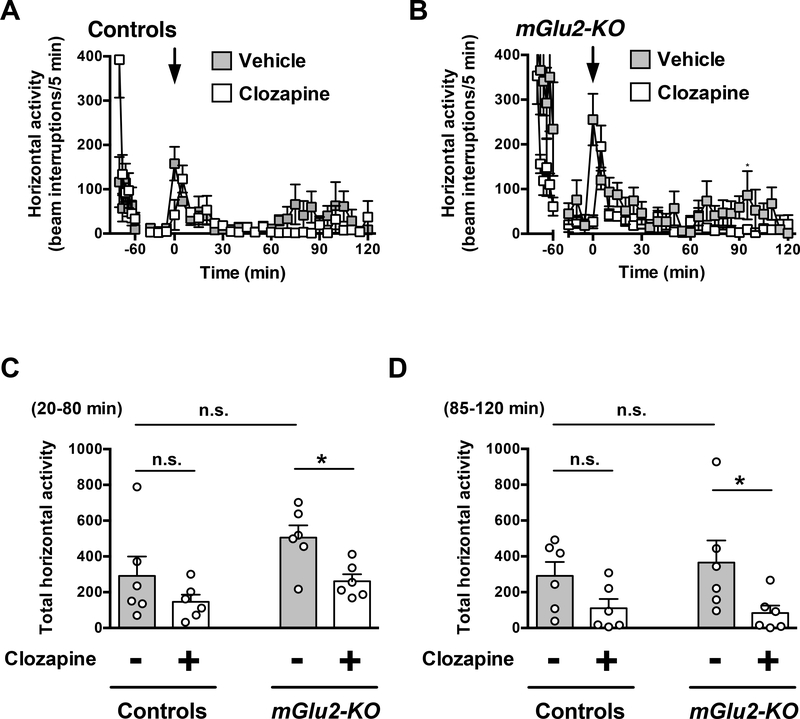
Two-way ANOVA revealed that there was not a significant effect of clozapine treatment on locomotor activity time course from t = 20 to t = 120 min in wild-type mice (Fig. 3A) (F1,10 = 3.55; P > 0.05). Two-way ANOVA revealed that there was a significant effect of clozapine treatment on locomotor activity time course from t = 20 to t = 120 min in mGlu2-KO mice (Fig. 3B) (F1,10 = 20.99; P < 0.01).
Fig. 3.
Effect of clozapine on locomotion. (A-D) Wild-type and mGlu2-KO mice mice were placed in the locomotor chamber and allowed to habituate for 90 min. After habituation, mice were injected with clozapine (1.5 mg/kg) or vehicle, and locomotor activity was measured for another 120 min (n = 6). (A,B) The panel depicts the time course of clozapine-modulated locomotion in 5 min blocks. Time of injection is indicated by arrow. (C,D) Data summary of the total clozapine-modulated locomotion as a summation of horizontal activity from t = 20 to t = 80 min (C) and from t = 85 to t = 120 min (D). *P < 0.05, ***P < 0.001, Bonferroni’s post hoc test of two-way ANOVA. Data are means ± SEM, n.s., not significant.
Two-way ANOVA also indicated that there was a significant effect of clozapine treatment on summation of locomotor activity from t = 20 to t = 80 min (Fig. 3C) (F1,20 = 7.75; P < 0.05). Similarly, two-way ANOVA revealed that there was a significant effect of genotype on summation of locomotor activity from t = 20 to t = 80 min (Fig. 3C) (F1,20 = 5.53; P < 0.05). Post hoc analysis indicated that clozapine decreased locomotor activity from t = 20 to t = 80 min in mGlu2-KO mice (P < 0.05), but not in wild-type mice (Fig. 3C) (P > 0.05). Post hoc analysis also showed absence of differences in locomotor activity from t = 20 to t = 80 min in vehicle-treated mGlu2-KO mice as compared to vehicle-treated wild-type mice (Fig. 3C) (P > 0.05).
Two-way ANOVA indicated that there was a significant effect of clozapine treatment on summation of locomotor activity from t = 85 to t = 120 min (Fig. 3D) (F1,20 = 8.30; P < 0.01). Two-way ANOVA revealed that there was not a significant effect of genotype on summation of locomotor activity from t = 85 to t = 120 min (Fig. 3D) (F1,20 = 0.08; P > 0.05). Post hoc analysis indicated that clozapine decreased locomotor activity from t = 85 to t = 120 min in mGlu2-KO mice (P < 0.05), but not in wild-type mice (Fig. 3D) (P > 0.05). Post hoc analysis also showed absence of differences in locomotor activity from t = 85 to t = 120 min in vehicle-treated mGlu2-KO mice as compared to vehicle-treated wild-type mice (Fig. 3D) (P > 0.05).
Effect of the clozapine on MK801-induced hyperlocomotor activity
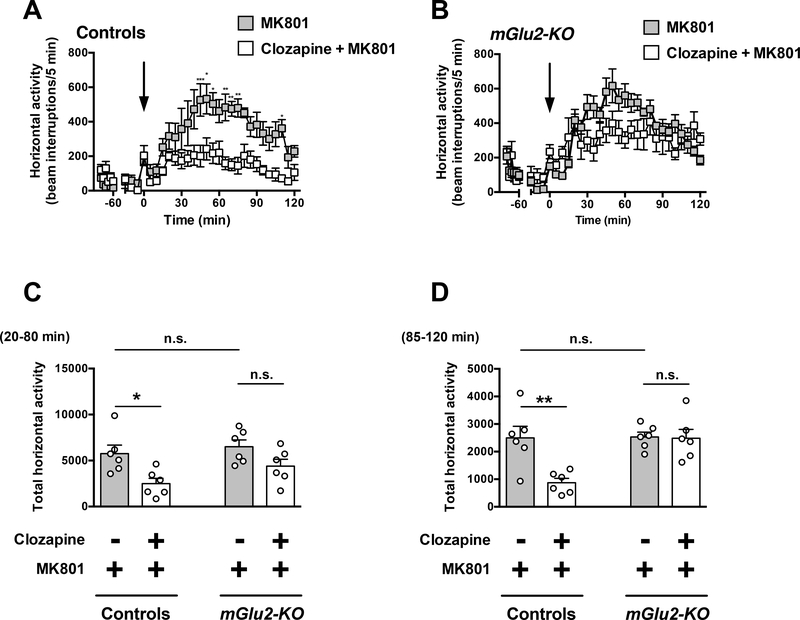
Two-way ANOVA revealed that there was a significant effect of clozapine treatment on MK801-induced hyperlocomotor activity time course from t = 20 to t = 120 min in wildtype mice (Fig. 4A) (F1,10 = 12.11; P < 0.01). However, two-way ANOVA revealed that there was not a significant effect of clozapine treatment on MK801-induced hyperlocomotor time course from t = 20 to t = 120 min in mGlu2-KO mice (Fig. 4B) (F1,10 = 3.49; P > 0.05).
Fig. 4.
Effect of clozapine on MK801-induced locomotion. (A-D) Wild-type and mGlu2KO mice were placed in the locomotor chamber and allowed to habituate for 90 min. After habituation, mice were injected with clozapine (1.5 mg/kg) or vehicle followed by MK801 (0.5 mg/kg), and locomotor activity was measured for another 120 min (n = 6). (A,B) The panel depicts the time course of MK801-induced locomotion in 5 min blocks. Time of injection is indicated by arrow. (C,D) Data summary of the total MK801-induced locomotion as a summation of horizontal activity from t = 20 to t = 80 min (C) and from t = 85 to t = 120 min (D). *P < 0.05, **P < 0.01, ***P < 0.001, Bonferroni’s post hoc test of two-way ANOVA. Data are means ± SEM, n.s., not significant.
Two-way ANOVA also indicated that there was a significant effect of clozapine treatment on summation of MK801-induced hyperlocomotor activity from t = 20 to t = 80 min (Fig. 4C) (F1,20 = 12.72; P < 0.01). Two-way ANOVA revealed that there was not a significant effect of genotype on summation of MK801-induced hyperlocomotor from t = 20 to t = 80 min (Fig. 4C) (F1,20 = 3.06; P > 0.05). Post hoc analysis indicated that clozapine decreased MK801-induced hyperlocomotor activity from t = 20 to t = 80 min in wild-type mice (P < 0.05), but not in mGlu2-KO mice (Fig. 4C) (P > 0.05). Post hoc analysis also showed absence of differences in locomotor activity from t = 20 to t = 80 min in mGlu2-KO mice treated with MK801 and vehicle as compared to wild-type mice treated with MK801 and vehicle (Fig. 4C) (P > 0.05).
Two-way ANOVA indicated that there was a significant effect of clozapine treatment on summation of MK801-induced hyperlocomotor activity from t = 85 to t = 120 min (Fig. 4D) (F1,20 = 8.28; P < 0.01). Two-way ANOVA revealed that there was a significant effect of genotype on summation of MK801-induced hyperlocomotor from t = 85 to t = 120 min (Fig. 4D) (F1,20 = 8.04; P < 0.05). Post hoc analysis indicated that clozapine decreased MK801-induced hyperlocomotor activity from t = 85 to t = 120 min in wildtype mice (P < 0.01), but not in mGlu2-KO mice (Fig. 4D) (P > 0.05). Post hoc analysis also showed absence of differences in locomotor activity from t = 85 to t = 120 min in mGlu2-KO mice treated with MK801 and vehicle as compared to wild-type mice treated with MK801 and vehicle (Fig. 4D) (P > 0.05).
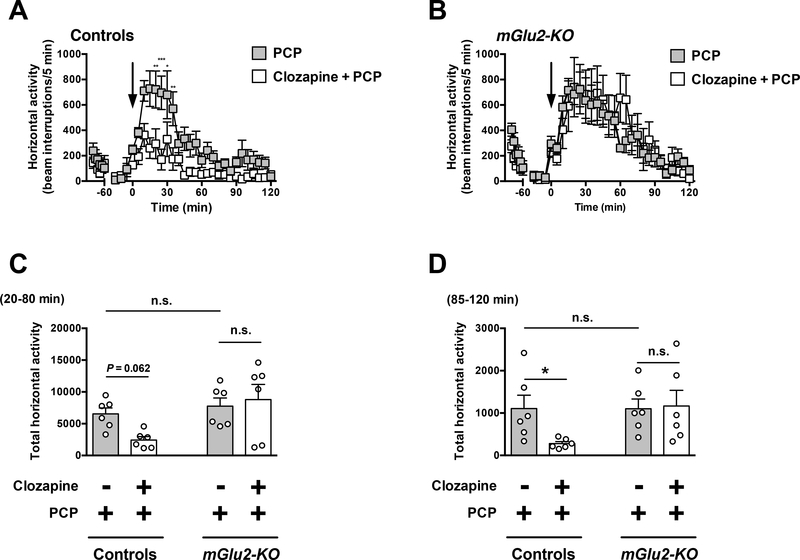
Effect of the clozapine on PCP-induced hyperlocomotor activity
Two-way ANOVA revealed that there was a significant effect of clozapine treatment on PCP-induced hyperlocomotor activity time course from t = 20 to t = 120 min in wild-type mice (Fig. 5A) (F1,10 = 26.84; P < 0.001). However, two-way ANOVA revealed that there was not a significant effect of clozapine treatment on PCP-induced hyperlocomotor time course from t = 20 to t = 120 min in mGlu2-KO mice (Fig. 5B) (F1,10 = 0.084; P > 0.05).
Fig. 5.
Effect of clozapine on PCP-induced locomotion. (A-D) Wild-type and mGlu2-KO mice were placed in the locomotor chamber and allowed to habituate for 90 min. After habituation, mice were injected with clozapine (1.5 mg/kg) or vehicle followed by PCP (7.5 mg/kg), and locomotor activity was measured for another 120 min (n = 6). (A,B) The panel depicts the time course of PCP-induced locomotion in 5 min blocks. Time of injection is indicated by arrow. (C,D) Data summary of the total PCP-induced locomotion as a summation of horizontal activity from t = 20 to t = 80 min (C) and from t = 85 to t = 120 min (D). *P < 0.05, **P < 0.01, ***P < 0.001, Bonferroni’s post hoc test of two-way ANOVA. Data are means ± SEM, n.s., not significant.
Two-way ANOVA also indicated that there was not a significant effect of clozapine treatment on summation of PCP-induced hyperlocomotor activity from t = 20 to t = 80 min (Fig. 5C) (F1,20 = 1.14; P > 0.05). Two-way ANOVA revealed that there was a significant effect of genotype on summation of PCP-induced hyperlocomotor activity from t = 20 to t = 80 min (Fig. 5C) (F1,20 = 6.71; P < 0.05). Post hoc analysis showed a trend for reduced PCP-induced hyperlocomotor activity from t = 20 to t = 80 min in wildtype mice treated with clozapine (Fig. 5C) (P = 0.062). Post hoc analysis showed absence of differences in PCP-induced hyperlocomotor activity from t = 20 to t = 80 min in mGlu2-KO mice treated with clozapine (Fig. 5C) (P > 0.05). Post hoc analysis also showed absence of differences in locomotor activity from t = 20 to t = 80 min in mGlu2KO mice treated with PCP and vehicle as compared to wild-type mice treated with PCP and vehicle (Fig. 5C) (P > 0.05).
Two-way ANOVA indicated that there was not a significant effect of clozapine treatment on summation of PCP-induced hyperlocomotor activity from t = 85 to t = 120 min (Fig. 5D) (F1,20 = 1.98; P > 0.05). Two-way ANOVA revealed that there was not a significant effect of genotype on summation of PCP-induced hyperlocomotor from t = 85 to t = 120 min (Fig. 5D) (F1,20 = 2.66; P > 0.05). However, post hoc analysis indicated that clozapine decreased PCP-induced hyperlocomotor activity from t = 85 to t = 120 min in wild-type mice (P < 0.05), but not in mGlu2-KO mice (Fig. 5D) (P > 0.05). Post hoc analysis also showed absence of differences in locomotor activity from t = 85 to t = 120 min in mGlu2-KO mice treated with PCP and vehicle as compared to wild-type mice treated with PCP and vehicle (Fig. 5D) (P > 0.05).
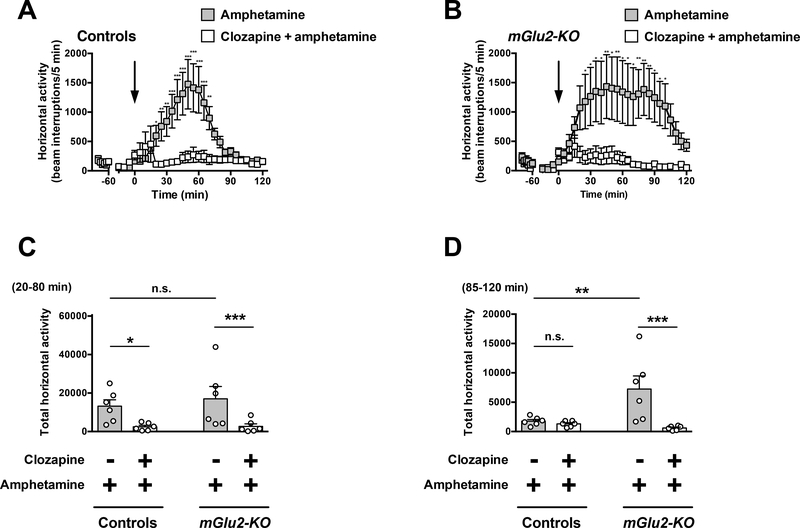
Effect of the clozapine on amphetamine-induced hyperlocomotor activity
Two-way ANOVA revealed that there was a significant effect of clozapine treatment on amphetamine-induced hyperlocomotor activity time course from t = 20 to t = 120 min in wild-type mice (Fig. 6A) (F1,10 = 10.72; P < 0.01). Two-way ANOVA also revealed that there was a significant effect of clozapine treatment on amphetamine-induced hyperlocomotor time course from t = 20 to t = 120 min in mGlu2-KO mice (Fig. 6B) (F1,10 = 5.80; P < 0.05).
Fig. 6.
Effect of clozapine on amphetamine-induced locomotion. (A-D) Wild-type and mGlu2-KO mice were placed in the locomotor chamber and allowed to habituate for 90 min. After habituation, mice were injected with clozapine (1.5 mg/kg) or vehicle followed by amphetamine (6.0 mg/kg), and locomotor activity was measured for another 120 min (n = 6). (A,B) The panel depicts the time course of amphetamine-induced locomotion in 5 min blocks. Time of injection is indicated by arrow. (C,D) Data summary of the total amphetamine-induced locomotion as a summation of horizontal activity from t = 20 to t = 80 min (C) and from t = 85 to t = 120 min (D). *P < 0.05, **P < 0.01, ***P < 0.001, Bonferroni’s post hoc test of two-way ANOVA. Data are means ± SEM, n.s., not significant.
Two-way ANOVA indicated that there was a significant effect of clozapine treatment on summation of amphetamine-induced hyperlocomotor activity from t = 20 to t = 80 min (Fig. 6C) (F1,20 = 11.57; P< 0.01). Two-way ANOVA also revealed that there was not a significant effect of genotype on summation of amphetamine-induced hyperlocomotor activity from t = 20 to t = 80 min (Fig. 6C) (F1,20 = 0.30; P > 0.05). Post hoc analysis indicated that clozapine decreased amphetamine-induced hyperlocomotor activity from t = 20 to t = 80 min in both wild-type (P < 0.05) and mGlu2-KO mice (Fig. 6C) (P < 0.05). Post hoc analysis also showed absence of differences in locomotor activity from t = 20 to t = 80 min in mGlu2-KO mice treated with amphetamine and vehicle as compared to wild-type mice treated with amphetamine and vehicle (Fig. 6C) (P > 0.05).
Two-way ANOVA indicated that there was a significant effect of clozapine treatment on summation of amphetamine-induced hyperlocomotor activity from t = 85 to t = 120 min (Fig. 6D) (F1,20 = 9.86; P < 0.01). Two-way ANOVA revealed that there was a significant effect of genotype on summation of amphetamine-induced hyperlocomotor from t = 85 to t = 120 min (Fig. 6D) (F1,20 = 4.44; P < 0.05). Post hoc analysis indicated that clozapine decreased amphetamine-induced hyperlocomotor activity from t = 85 to t = 120 min in mGlu2-KO mice (Fig. 6D) (P < 0.001), but not in wild-type mice (Fig. 6D) (P > 0.05). Post hoc analysis also showed augmented hyperlocomotor activity from t = 85 to t = 120 min in mGlu2-KO mice treated with amphetamine and vehicle as compared to wild-type mice treated with amphetamine and vehicle (Fig. 6D) (P < 0.01).
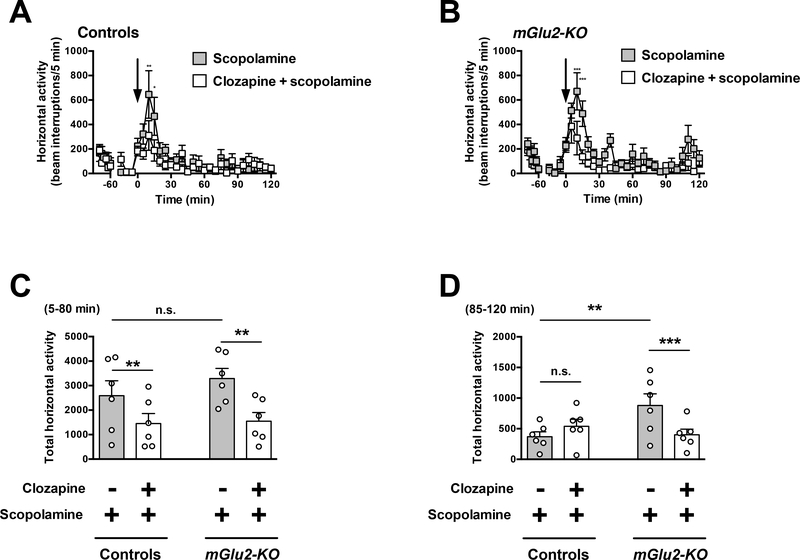
Effect of the clozapine on scopolamine-induced hyperlocomotor activity
Two-way ANOVA revealed that there was a significant effect of clozapine treatment on scopolamine-induced hyperlocomotor activity time course from t = 5 to t = 120 min in wild-type mice (Fig. 7A) (F1,10 = 5.90; P < 0.05). Two-way ANOVA revealed that there was a significant effect of clozapine treatment on scopolamine-induced hyperlocomotor time course from t = 5 to t = 120 min in mGlu2-KO mice (Fig. 7B) (F1,10 = 12.94; P < 0.01).
Fig. 7.
Effect of clozapine on scopolamine-induced locomotion. (A-D) Wild-type and mGlu2-KO mice were placed in the locomotor chamber and allowed to habituate for 90 min. After habituation, mice were injected with clozapine (1.5 mg/kg) or vehicle followed by scopolamine (2.0 mg/kg), and locomotor activity was measured for another 120 min (n = 6). (A,B) The panel depicts the time course of scopolamine-induced locomotion in 5 min blocks. Time of injection is indicated by arrow. (C,D) Data summary of the total scopolamine-induced locomotion as a summation of horizontal activity from t = 5 to t = 80 min (C) and from t = 85 to t = 120 min (D). *P < 0.05, **P < 0.01, ***P < 0.001, Bonferroni’s post hoc test of two-way ANOVA. Data are means ± SEM, n.s., not significant.
Two-way ANOVA also indicated that there was a significant effect of clozapine treatment on summation of scopolamine-induced hyperlocomotor activity from t = 5 to t = 80 min (Fig. 7C) (F1,20 = 10.01; P < 0.01). Two-way ANOVA revealed that there was not a significant effect of genotype on summation of amphetamine-induced hyperlocomotor activity from t = 5 to t = 80 min (Fig. 7C) (F1,20 = 0.76; P > 0.05). Post hoc analysis indicated that clozapine decreased scopolamine-induced hyperlocomotor activity from t = 5 to t = 80 min in both wild-type (P < 0.01) and mGlu2-KO mice (Fig. 7C) (P< 0.01). Post hoc analysis also showed absence of differences in locomotor activity from t = 5 to t = 80 min in mGlu2-KO mice treated with scopolamine and vehicle as compared to wild-type mice treated with scopolamine and vehicle (Fig. 7C) (P > 0.05).
Two-way ANOVA indicated that there was not a significant effect of clozapine treatment on summation of scopolamine-induced hyperlocomotor activity from t = 85 to t = 120 min (Fig. 7D) (F1,20 = 1.48; P > 0.05). Two-way ANOVA revealed that there was a significant effect of genotype on summation of scopolamine-induced hyperlocomotor from t = 85 to t = 120 min (Fig. 7D) (F1,20 = 2.13; P < 0.05). Post hoc analysis indicated that clozapine decreased scopolamine-induced hyperlocomotor activity from t = 85 to t = 120 min in mGlu2-KO mice (Fig. 7D) (P < 0.001), but not in wild-type mice (Fig. 7D) (P > 0.05). Post hoc analysis also showed augmented hyperlocomotor activity from t = 85 to t = 120 min in mGlu2-KO mice treated with scopolamine and vehicle as compared to wild-type mice treated with scopolamine and vehicle (Fig. 7D) (P < 0.01).
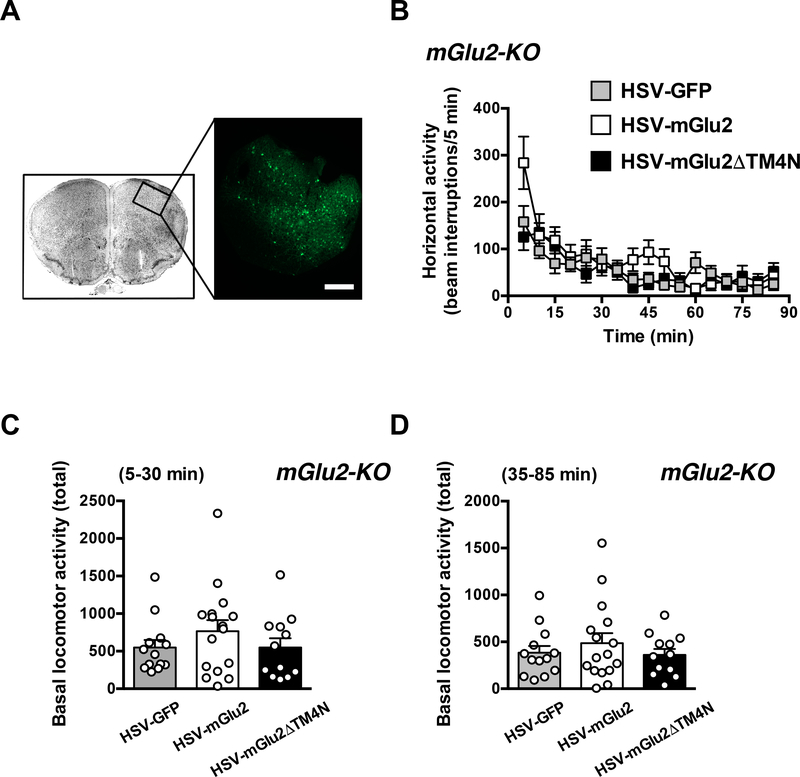
Virally mediated over-expression of mGlu2 in the frontal cortex of mGlu2-KO mice does not affect exploratory behavior
mGlu2-KO mice received intra-frontal cortical injections of HSV expressing GFP alone or either mGlu2 or mGlu2DTM4N together with GFP. Immunofluorescence assays confirmed that the viral vector was injected into the appropriate brain region (Fig. 8A).
Fig. 8.
Virus-mediated over-expression of mGlu2 or HSV-mGlu2ΔTM4 in the frontal cortex of mGlu2-KO mice. (A) HSV-GFP was injected into the frontal cortex, and GFP expression was revealed by immunohistochemistry. Scale bar, 200 μm. (B-D) Virusmediated over-expression of mGlu2 or HSV-mGlu2ΔTM4 in the frontal cortex of mGlu2KO mice does not affect exploratory behavior. HSV-GFP, HSV-mGlu2 or HSVmGlu2ΔTM4 were injected into the frontal cortex of mGlu2-KO mice. Animals were placed in the locomotor chamber, and allowed to habituate for 5 min. After habituation, horizontal activity (n = 6) was measured for 85 min. Time courses (B) and total counts from t = 5 to t = 35 min (C) and from t = 40 to t = 85 min (D) are shown for each measurement. Bonferroni’s post hoc test of one-way ANOVA. Data are means ± SEM.
Two-way ANOVA revealed that there was not significant effect of viral injection on spontaneous locomotor activity (exploratory behavior) in mGlu2-KO mice (Fig. 8B) (F2,38 = 0.96; P > 0.05). One-way ANOVA indicated that there was not a significant effect of viral injection on summation of horizontal activity from t = 5 to t = 35 min in mGlu2-KO mice (Fig. 8C) (F2,38 = 0.90; P > 0.05). One-way ANOVA indicated that there was not a significant effect of viral injection on summation of horizontal activity from t = 40 to t = 85 min in mGlu2-KO mice (Fig. 8D) (F2,38 = 0.61; P > 0.05).
Virally mediated over-expression of mGlu2 in the frontal cortex of mGlu2-KO mice rescues antipsychotic-like behavior
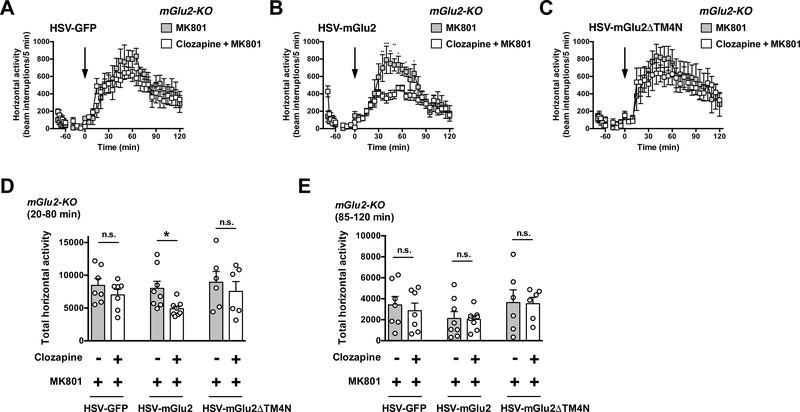
Two-way ANOVA indicated that there was not a significant effect of clozapine treatment on MK801-induced hyperlocomotor activity time course from t = 20 to t = 120 min in mGlu2-KO mice injected with HSV-GFP (Fig. 9A) (F1,12 = 1.01; P > 0.05). Two-way ANOVA revealed that there was a significant effect of clozapine treatment on MK801induced hyperlocomotor activity time course from t = 20 to t = 120 min in mGlu2-KO mice injected with HSV-mGlu2 (Fig. 9B) (F1,14 = 7.42; P < 0.05). Two-way ANOVA indicated that there was not a significant effect of clozapine treatment on MK801induced hyperlocomotor activity time course from t = 20 to t = 120 min in mGlu2-KO mice injected with HSV-mGluΔDTM4 (Fig. 9C) (F1,10 = 0.18; P > 0.05).
Fig. 9.
Virally mediated over-expression of mGlu2, but not mGlu2DTM4, in the frontal cortex of mGlu2-KO mice rescues the effect of clozapine on MK801-induced hyperlocomotor activity. (A-E) HSV-GFP, HSV-mGlu2 or HSV-mGlu2DTM4 were injected into the frontal cortex of mGlu2-KO mice. Animals were placed in the locomotor chamber and allowed to habituate for 90 min. After habituation, mice were injected with clozapine (1.5 mg/kg) or vehicle followed by MK801 (0.5 mg/kg), and locomotor activity was measured for another 120 min (n = 7–8). (A-C) The panel depicts the time course of MK801-induced locomotion in 5 min blocks. Time of injection is indicated by arrow. (D,E) Data summary of the total MK801-induced locomotion as a summation of horizontal activity from t = 20 to t = 80 min (D) and from t = 85 to t = 120 min (E). *P < 0.05, **P < 0.01, ***P < 0.001, Bonferroni’s post hoc test of two-way ANOVA. Data are means ± SEM, n.s., not significant.
Two-way ANOVA indicated that there was a significant effect of clozapine treatment on summation of MK801-induced hyperlocomotor activity from t = 20 to t = 80 min (Fig. 9D) (F1,36 = 4.95; P < 0.05). Two-way ANOVA also revealed that there was not a significant effect of viral injection on summation of MK801-induced hyperlocomotor from t = 20 to t = 80 min (Fig. 9D) (F2,36 = 1.48; P > 0.05). Post hoc analysis indicated that clozapine decreased MK801-induced hyperlocomotor activity from t = 20 to t = 80 min in mGlu2KO mice stereotaxically injected with HSV-mGlu2 (P < 0.05), but not with HSV-GFP (P > 0.05) or HSV-mGlu2DTM4 (P > 0.05) (Fig. 9D).
Two-way ANOVA indicated that there was not a significant effect of clozapine treatment on summation of MK801-induced hyperlocomotor activity from t = 85 to t = 120 min (Fig. 9E) (F1,36 = 0.17; P > 0.05). Two-way ANOVA revealed that there was not a significant effect of viral injection on summation of MK801-induced hyperlocomotor from t = 85 to t = 120 min (Fig. 9E) (F2,36 = 2.28; P > 0.05). Post hoc analysis indicated that clozapine did not affect MK801-induced hyperlocomotor activity from t = 85 to t = 120 min in mGlu2-KO mice stereotaxically injected with HSV-GFP (P > 0.05), HSV-mGlu2 (P > 0.05) or HSV-mGluΔDTM4 (P > 0.05) (Fig. 9E).
Discussion
We tested the extent to which mGlu2 receptor expression is necessary for the effects of the atypical antipsychotic clozapine on prevention of hyperlocomotor activity induced by different families of psychoactive substances – these included the dissociative drugs MK801 and PCP, the stimulant drug amphetamine, and the deliriant drug scopolamine. We show that the inhibitory effect of clozapine treatment on either MK801-induced or PCP-induced hyperlocomotor activity was observed in wild-type mice, but was absent in mGlu2-KO mice. Notably, this need for mGlu2 receptor expression to induce clozapinedependent antipsychotic-related effects was not observed in mice injected with amphetamine or scopolamine. Additionally, virally mediated over-expression of mGlu2 in the frontal cortex of mGlu2-KO mice was sufficient to rescue the preventive effect of clozapine administration on MK801-induced hyperlocomotor activity.
Animals with a high level of anxiety or very low levels of activity tend to remain in the periphery of a testing enclosure (Weisstaub et al. 2006). Conversely, animals with low levels of anxiety generally spend greater amounts of time in the center “unprotected” area. Thus, measures of total distance covered during locomotion are used as an index of exploratory activity, while the proportion of time spent in the center of the arena is taken as a measure of anxiety. In agreement with previous reports (Morishima et al. 2005), our data demonstrate that exploratory activity (total distance) was increased in mGlu2-KO mice as compared to wild-type littermates. We also found that mGlu2-KO mice explored the center portion of the environment (as a percentage of total exploratory time) more than their control littermates. These findings suggest that global disruption of mGlu2 receptor-dependent signaling reduces inhibition in conflict anxiety paradigms.
Previous reports have described that the 5-HT2A/2C receptor agonist DOI induces opposing effects on locomotor activity in mice (Halberstadt et al. 2009). Thus, low doses of DOI increase locomotor activity, an effect absent in 5-HT2A-KO mice, whereas higher doses of DOI decrease locomotor activity in a manner prevented by the 5-HT2C antagonist SER-082. These changes in locomotor activity were observed in mice that were placed in the open field 15 min after treatment with DOI (Halberstadt et al. 2009). We show that, as expected, exploratory activity correlates negatively with time of exposure: exploratory behavior was almost null after 60 min of exposure to the open field. Nevertheless, DOI administration (1.0 mg/kg) injected 90 min after habituation to the open field still moderately increased locomotor activity in wild-type mice. This further demonstrates that activation of the 5-HT2A receptor increases locomotor activity in rodent models. Additionally, this effect of DOI augmenting locomotor activity and exploratory behavior was not observed in mGlu2-KO mice, which further suggests that mGlu2 plays a fundamental role in modulating 5-HT2A receptor-dependent phenotypes. Although post hoc analysis showed absence of differences in locomotor activity between vehicle-treated wild-type mice and vehicle-treated mGlu2-KO mice (see Fig. 2C and Fig. 2D), an alternative explanation for the absence of effect of DOI administration on locomotor activity in mGlu2-KO mice as compared to mGlu2-KO mice treated with vehicle may be related to the trend toward increased exploratory behavior in mGlu2-KO mice together with the relatively moderate effect of DOI on locomotor activity.
Our current findings suggest that, as expected, administration of the atypical antipsychotic clozapine decreases the hyperlocomotor activity induced by the psychoactive drugs MK801, PCP, amphetamine and scopolamine in wild-type mice. Because clozapine binds with high affinity to the 5-HT2A receptor, and with lower affinity to dopamine D2 and other monoaminergic receptors (Miyamoto et al. 2012), we and others have previously established the lowest dose of clozapine that reduces MK801induced hyperlocomotor activity in mice via interaction with the 5-HT2A receptor (Fribourg et al. 2011; Schmid et al. 2014). Thus, we showed that pretreatment with clozapine (1.5 mg/kg) significantly decreases MK801-induced hyperlocomotor activity in wild-type mice, but not in 5-HT2A-KO littermates (Fribourg et al. 2011). Administration of higher doses of clozapine (10 mg/kg) is able to suppress MK801-induced hyperlocomotor activity in both wild-type and 5-HT2A-KO mice (Fribourg et al. 2011). Our data here showing absence of effect with low doses of clozapine (1.5 mg/kg) on locomotion in wild-type mice are consonant with previous reports suggesting that low doses of clozapine (below 3 mg/kg) do not significantly decrease locomotion in mice (McOmish et al. 2012). Importantly, our data also suggest the pivotal finding that the effect of intraperitoneal administration of clozapine (1.5 mg/kg) on hyperlocomotor activity induced by the dissociative drugs MK801 or PCP is significantly diminished in mice with global disruption of mGlu2 receptor-dependent signaling. However, this effect of clozapine treatment on suppression of hyperlocomotor activity was present when mGlu2-KO mice were injected with either amphetamine or scopolamine. Together, these data suggest that expression of mGlu2 receptor is necessary for the 5-HT2A receptor-dependent effects of clozapine on hyperlocomotor activity induced by dissociative drugs whose primary action relies on disruption of glutamate neurotransmission.
A potential explanation for the effects of clozapine treatment on hyperlocomotor activity in mGlu2-KO mice injected with either amphetamine or scopolamine may be related to differences in specific signaling pathways or brain regions affected by disruptions of dopaminergic and cholinergic neurotransmission. Nonetheless, the precise molecular mechanism that results in differential modulation of hyperlocomotor activity phenotypes will require further investigation. We also found that the hyperlocomotive effect of amphetamine and scopolamine on hyperlocomotor activity was increased in mGlu2-KO mice as compared to wild-type controls. This particular phenotype, which was observed approximately one hour after administration of either amphetamine or scopolamine, may be influenced by the same mechanism underlying the augmented exploratory behavior observed in mGlu2 null-mutant mice that we speculate might be related to diminished modulation of glutamatergic signal upon deletion of mGlu2 expression. Additional work will be required to test the extent to which mGlu2 receptor-dependent signaling is needed for clozapine-dependent antipsychotic-like effect in additional behavioral models related to cognition, memory processing and sensorimotor gating. Similarly, considering that clozapine presents unique characteristics in both clinical efficacy and pharmacological properties at the 5-HT2A receptor (Meltzer 2013), further work will be needed to clarify whether other atypical antipsychotic drugs, such as olanzapine and risperidone, possess similar properties to those of clozapine targeting the 5-HT2A receptor.
The 5-HT2A receptor is widely expressed in CNS structures involved in cognition, perception and mood, such as the cerebral cortex, ventral striatum and ventrobasal thalamus (Willins et al. 1997; Jakab and Goldman-Rakic 1998; Lopez-Gimenez et al. 2001; Weber and Andrade 2010). Using a Cre-loxP system to drive selective cortical expression of the 5-HT2A receptor, our previous findings suggested that cortical glutamatergic, but not sub-cortical, 5-HT2A receptor expression, is necessary to mediate the signaling pattern and psychosis-like behavioral effects of hallucinogenic 5-HT2A receptor agonists (Gonzalez-Maeso et al. 2007). It has also been shown that mGlu2 receptor is located both postsynaptically as well as presynaptically in glutamatergic terminals in the frontal cortex (Neki et al. 1996; Ohishi et al. 1998; Moreno et al. 2016). However, the role of cortical mGlu2 in the therapeutic-related behavioral effects of clozapine treatment remained poorly characterized. Using a virally mediated overexpression approach, our data suggest that frontal cortex neurons may be unique in their capacity to trigger the specific signaling pathways that prevent hyperlocomotor activity induced by dissociative drugs. Thus, our data show that the effect of clozapine on prevention of MK801-induced hyperlocomotor activity is rescued in mGlu2-KO mice stereotaxically injected with HSV-mGlu2 in the frontal cortex, an effect not observed in mGlu2-KO mice stereotaxically injected with HSV-GFP. Although these data suggest that postsynaptic mGlu2 is necessary for the effects of clozapine treatment on prevention of hyperlocomotor activity induced by MK801 treatment, further work will be needed with different viral vectors expressing mGlu2 under the control of different promoters to rescue mGlu2 expression at either postsynaptic or presynaptic sites in order to provide a better understanding of the cell type and neural circuits responsible for these antipsychotic-related phenotypes.
Family A GPCRs, such as serotonin, norepinephrine and opioid receptors, were assumed to function as strict monomers. This observation is supported by previous findings that showed functional receptor-G protein coupling of a single family A GPCR, such as β2-adrenergic receptor (Whorton et al. 2007), rhodopsin (Whorton et al. 2008) or the μ-opioid receptor (Kuszak et al. 2009), reconstituted in nanodiscs. This is consistent with recent X-ray crystal (Rasmussen et al. 2011) and cryo-EM GPCR-G protein complex (Thomsen et al. 2016; Liang et al. 2017) structures. Nevertheless, many instances of homomerization and heteromerization have been reported among different GPCR families, demonstrating that GPCR homo- and heteromerization dramatically affect receptor trafficking, pharmacology and function (Ward et al. 2013; Ferre et al. 2014). Most of these studies, however, have been reported in heterologous expression systems (González-Maeso 2011; Gonzalez-Maeso 2014), and hence only a limited number of reports can support the conclusion that GPCR complexes may play a role in physiological or behavioral phenotypes in rodent models. Our previous findings suggest that 5-HT2A and mGlu2 receptors are assembled as a GPCR heteromeric complex (Gonzalez-Maeso et al. 2008; Fribourg et al. 2011; Moreno et al. 2012; Baki et al. 2016; Moreno et al. 2016). This was demonstrated based on the use of biophysical approaches such as co-immunoprecipitation, bioluminescence resonance energy transfer (BRET), and fluorescence resonance energy transfer (FRET) in mammalian cell tissue culture. The physiological validity of these findings obtained in heterologous expression systems was corroborated by ex vivo assays, such as co-immunoprecipitation and sub-cellular proximity at the electron microscopy level in mouse and postmortem human frontal cortex. Although these findings support that 5HT2A and mGlu2 form part of the same protein complex, whether this GPCR heteromer plays a role in the behavioral phenotypes induced by antipsychotics remains unclear.
Within this context, our previous findings suggested that that three residues located at the intracellular end of transmembrane four (TM4) are responsible for the location of the mGlu2 receptor in close proximity to the 5-HT2A receptor in heterologous expression systems (Moreno et al. 2012). This was assessed by testing receptor heteromerization in living mammalian cells co-expressing the 5-HT2A receptor together with mGlu2 or different mGlu2/mGlu3 chimeric constructs, including mGlu2ΔDTM4N (Moreno et al. 2012). Considering that HSV-mediated over-expression of mGlu2, but not the mGlu2ΔDTM4N chimeric construct, in the frontal cortex of mGlu2-KO mice rescued the antipsychotic-related properties of clozapine, these data suggest that frontal cortex GPCR heteromeric assembly between 5-HT2A and mGlu2 receptors may be necessary for at least some of the antipsychotic-related behavioral effects of clozapine. An alternative, although not mutually exclusive, explanation for the absence of effect of clozapine on MK801-induced hyperlocomotor activity observed in mGlu2-KO mice stereotaxically injected with either HSV-GFP or HSV-mGlu2ΔTM4N, but not with HSVmGlu2, might be related to molecular mechanisms involving the capability of the mGlu2ΔTM4N construct to functionally interact with other GPCRs or signaling proteins that affect 5-HT2A receptor expression, sub-cellular localization, trafficking and/or signaling, as well as the stoichiometry of these neurotransmitter receptors. Further work is therefore necessary to fully define whether a physical interaction between 5-HT2A and mGlu2 is needed for their pharmacological and behavioral crosstalk in whole animal model systems.
It is well recognized that sex differences have an impact on mental health and, in particular, on the course of schizophrenia (Crawford and DeLisi 2016). Considering that the current analysis focuses on male mice, future work should extend these findings to females to determine the generalizability and specificity of basic signaling mechanisms underlying hallucinogen and antipsychotic drug action.
In summary, we showed that absence of mGlu2 receptor-dependent signaling differentially affects the response to drugs bearing distinct mechanism of action yet commonly used as models of psychosis in mice. Additionally, we demonstrated that the effect of pretreatment with clozapine on hyperlocomotor activity induced by the dissociative drugs MK801 and PCP is reduced in mGlu2-KO mice, whereas clozapine administration was able to reduce hyperlocomotor activity induced by amphetamine and scopolamine in both mGlu2-KO and wild-type mice. Our data also suggest that virally mediated restoration of mGlu2 expression in the frontal cortex of mGlu2-KO mice rescues the repressive effect of clozapine treatment on MK801-induced hyperlocomotor activity. Together, these findings extend our knowledge of the role of mGlu2 receptordependent signaling in antipsychotic-related behavioral phenotypes in rodent models.
Acknowledgements
This study was supported by NIH grants R01-MH084894 (J.G.M.) R01-MH111940 (J.G.M.) and P50-AA022537 (M.F.M.).
Footnotes
Conflict of interest
The authors have not conflict of interest to report.
Compliance with ethical standards
Experiments were conducted in accord with NIH guidelines, and were approved by the Virginia Commonwealth University Animal Care and Use Committee.
References
- Agid O, Kapur S, Arenovich T, Zipursky RB (2003). Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry 60: 1228–1235 [DOI] [PubMed] [Google Scholar]
- Arguello PA, Gogos JA (2006). Modeling madness in mice: one piece at a time. Neuron 52: 179–196 [DOI] [PubMed] [Google Scholar]
- Baki L, Fribourg M, Younkin J, Eltit JM, Moreno JL, Park G, Vysotskaya Z, Narahari A, Sealfon SC, Gonzalez-Maeso J, Logothetis DE (2016). Cross-signaling in metabotropic glutamate 2 and serotonin 2A receptor heteromers in mammalian cells. Pflugers Arch 468: 775–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenga MJ, Chaney SF, Baez M, Britton TC, Hornback WJ, Monn JA, Marek GJ (2018). Metabotropic Glutamate2 Receptors Play a Key Role in Modulating Head Twitches Induced by a Serotonergic Hallucinogen in Mice. Front Pharmacol 9: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A, Willins DL, Gray JA, Woods J, Benovic JL, Roth BL (2001). The dynamindependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J Biol Chem 276: 8269–8277 [DOI] [PubMed] [Google Scholar]
- Bradford AM, Savage KM, Jones DN, Kalinichev M (2010). Validation and pharmacological characterisation of MK-801-induced locomotor hyperactivity in BALB/C mice as an assay for detection of novel antipsychotics. Psychopharmacology (Berl) 212: 155–170 [DOI] [PubMed] [Google Scholar]
- Bramness JG, Gundersen OH, Guterstam J, Rognli EB, Konstenius M, Loberg EM, Medhus S, Tanum L, Franck J (2012). Amphetamine-induced psychosis--a separate diagnostic entity or primary psychosis triggered in the vulnerable? BMC Psychiatry 12: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford MB, DeLisi LE (2016). Issues related to sex differences in antipsychotic treatment. Curr Opin Psychiatry 29: 211–217 [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH (2010). Interaction of 5-HT2A and 5-HT2C receptors in R(−)-2,5-dimethoxy-4iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther 335: 728734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, Kapur S, Fletcher PJ (2007). The amphetamine-induced sensitized state as a model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 31: 1556–1571 [DOI] [PubMed] [Google Scholar]
- Fell MJ, Svensson KA, Johnson BG, Schoepp DD (2008). Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane- 4,6-dicarboxylic acid (LY404039). J Pharmacol Exp Ther 326: 209–217 [DOI] [PubMed] [Google Scholar]
- Fernando AB, Robbins TW (2011). Animal Models of Neuropsychiatric Disorders. Annu Rev Clin Psychol [DOI] [PubMed] [Google Scholar]
- Ferre S, Casado V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin JP, Guitart X (2014). G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev 66: 413–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adney SK, Hatcher C, Eltit JM, Ruta JD, Albizu L, Li Z, Umali A, Shim J, Fabiato A, Mackerell AD, Jr., Brezina V, Sealfon SC, Filizola M, Gonzalez-Maeso J, Logothetis DE (2011). Decoding the Signaling of a GPCR Heteromeric Complex Reveals a Unifying Mechanism of Action of Antipsychotic Drugs. Cell 147: 1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J (2011). GPCR oligomers in pharmacology and signaling. Mol Brain 4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J (2014). Family a GPCR heteromers in animal models. Front Pharmacol 5: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC (2008). Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452: 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007). Hallucinogens Recruit Specific Cortical 5-HT(2A) Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 53: 439452. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC (2003). Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci 23: 8836–8843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, Powell SB (2009). 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology 34: 1958–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham S, Kim TK, Chung S, Im HI (2017). Drug Abuse and Psychosis: New Insights into Druginduced Psychosis. Exp Neurobiol 26: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks JB, Gonzalez-Maeso J (2013). Animal models of serotonergic psychedelics. ACS Chem Neurosci 4: 33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR (2000) Comparative cytoarchitectonic atlas of the C57BL/6 and 129/Sv mouse brains Elsevier, Elsevier [Google Scholar]
- Ibi D, de la Fuente Revenga M, Kezunovic N, Muguruza C, Saunders JM, Gaitonde SA, Moreno JL, Ijaz MK, Santosh V, Kozlenkov A, Holloway T, Seto J, Garcia-Bea A, Kurita M, Mosley GE, Jiang Y, Christoffel DJ, Callado LF, Russo SJ, Dracheva S, Lopez-Gimenez JF, Ge Y, Escalante CR, Meana JJ, Akbarian S, Huntley GW, Gonzalez-Maeso J (2017). Antipsychotic-induced Hdac2 transcription via NF-kappaB leads to synaptic and cognitive side effects. Nat Neurosci 20: 1247–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS (1998). 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A 95: 735–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, Watson DJ, Fone KC (2011). Animal models of schizophrenia. Br J Pharmacol 164: 1162–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita M, Holloway T, Garcia-Bea A, Kozlenkov A, Friedman AK, Moreno JL, Heshmati M, Golden SA, Kennedy PJ, Takahashi N, Dietz DM, Mocci G, Gabilondo AM, Hanks J, Umali A, Callado LF, Gallitano AL, Neve RL, Shen L, Buxbaum JD, Han MH, Nestler EJ, Meana JJ, Russo SJ, Gonzalez-Maeso J (2012). HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat Neurosci 15: 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuszak AJ, Pitchiaya S, Anand JP, Mosberg HI, Walter NG, Sunahara RK (2009). Purification and functional reconstitution of monomeric mu-opioid receptors: allosteric modulation of agonist binding by Gi2. J Biol Chem 284: 26732–26741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavreysen H, Langlois X, Donck LV, Nunez JM, Pype S, Lutjens R, Megens A (2015). Preclinical evaluation of the antipsychotic potential of the mGlu2-positive allosteric modulator JNJ-40411813. Pharmacol Res Perspect 3: e00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YL, Khoshouei M, Radjainia M, Zhang Y, Glukhova A, Tarrasch J, Thal DM, Furness SGB, Christopoulos G, Coudrat T, Danev R, Baumeister W, Miller LJ, Christopoulos A, Kobilka BK, Wootten D, Skiniotis G, Sexton PM (2017). Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature 546: 118–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, Marx CE, Aprille JR, Dwyer DS, Li XM, Mahadik SP, Duman RS, Porter JH, Modica-Napolitano JS, Newton SS, Csernansky JG (2008). Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev 60: 358–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Vilaro MT, Palacios JM, Mengod G (2001). Mapping of 5-HT2A receptors and their mRNA in monkey brain: [3H]MDL100,907 autoradiography and in situ hybridization studies. J Comp Neurol 429: 571–589 [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A (1997). Ketamineinduced exacerbation of psychotic symptoms and cognitive impairment in neurolepticfree schizophrenics. Neuropsychopharmacology 17: 141–150 [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK (2000). Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther 292: 76–87 [PubMed] [Google Scholar]
- McOmish CE, Lira A, Hanks JB, Gingrich JA (2012). Clozapine-induced locomotor suppression is mediated by 5-HT(2A) receptors in the forebrain. Neuropsychopharmacology 37: 2747–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY (2013). Update on typical and atypical antipsychotic drugs. Annu Rev Med 64: 393–406 [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA (2012). Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry 17: 1206–1227 [DOI] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J (2011). Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett 493: 76–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Umali A, Rayannavar V, Sealfon SC, Gonzalez-Maeso J (2013). Persistent effects of chronic clozapine on the cellular and behavioral responses to LSD in mice. Psychopharmacology (Berl) 225: 217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Miranda-Azpiazu P, Garcia-Bea A, Younkin J, Cui M, Kozlenkov A, Ben-Ezra A, Voloudakis G, Fakira AK, Baki L, Ge Y, Georgakopoulos A, Moron JA, Milligan G, Lopez-Gimenez JF, Robakis NK, Logothetis DE, Meana JJ, Gonzalez-Maeso J (2016). Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Sci Signal 9: ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Muguruza C, Umali A, Mortillo S, Holloway T, Pilar-Cuellar F, Mocci G, Seto J, Callado LF, Neve RL, Milligan G, Sealfon SC, Lopez-Gimenez JF, Meana JJ, Benson DL, Gonzalez-Maeso J (2012). Identification of three residues essential for 5hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A.mGlu2) receptor heteromerization and its psychoactive behavioral function. J Biol Chem 287: 4430144319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y, Miyakawa T, Furuyashiki T, Tanaka Y, Mizuma H, Nakanishi S (2005). Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci U S A 102: 4170–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neki A, Ohishi H, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N (1996). Pre- and postsynaptic localization of a metabotropic glutamate receptor, mGluR2, in the rat brain: an immunohistochemical study with a monoclonal antibody. Neurosci Lett 202: 197–200 [DOI] [PubMed] [Google Scholar]
- Nichols DE (2004). Hallucinogens. Pharmacol Ther 101: 131–181 [DOI] [PubMed] [Google Scholar]
- Nichols DE (2016). Psychedelics. Pharmacol Rev 68: 264–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi H, Neki A, Mizuno N (1998). Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci Res 30: 65–82 [DOI] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stampfli P, Liechti ME, Seifritz E, Vollenweider FX (2017). The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr Biol 27: 451–457 [DOI] [PubMed] [Google Scholar]
- Raote I, Bhattacharyya S, Panicker MM (2013). Functional Selectivity in Serotonin Receptor 2A (5-HT2A) Endocytosis, Recycling, and Phosphorylation. Mol Pharmacol 83: 42–50 [DOI] [PubMed] [Google Scholar]
- Rasmussen SGF, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah STa, Lyons Ja, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK (2011). Crystal structure of the β2 adrenergic receptor–Gs protein complex. Nature [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Streicher JM, Meltzer HY, Bohn LM (2014). Clozapine acts as an agonist at serotonin 2A receptors to counter MK-801- induced behaviors through a betaarrestin2independent activation of Akt. Neuropsychopharmacology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, Brenneisen R, Muller F, Borgwardt S, Liechti ME (2015). Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biol Psychiatry [DOI] [PubMed] [Google Scholar]
- Steeds H, Carhart-Harris RL, Stone JM (2015). Drug models of schizophrenia. Ther Adv Psychopharmacol 5: 43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LC, Clarke WP, Berg KA (2015). Atypical antipsychotics and inverse agonism at 5-HT2 receptors. Curr Pharm Des 21: 3732–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen AR, Plouffe B, Cahill TJ, 3rd, Shukla AK, Tarrasch JT, Dosey AM, Kahsai AW, Strachan RT, Pani B, Mahoney JP, Huang L, Breton B, Heydenreich FM, Sunahara RK, Skiniotis G, Bouvier M, Lefkowitz RJ(2016). GPCR-G Protein-beta-Arrestin Super-Complex Mediates Sustained G Protein Signaling. Cell 166: 907–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht D, Koller R, Vollenweider FX, Schmid L (2002). Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biological psychiatry 51: 400–406 [DOI] [PubMed] [Google Scholar]
- Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC (2000). Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Archives of general psychiatry 57: 1139–1147 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D (1998). Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9: 3897–3902 [DOI] [PubMed] [Google Scholar]
- Ward RJ, Xu TR, Milligan G (2013). GPCR Oligomerization and Receptor Trafficking. Methods Enzymol 521: 69–90 [DOI] [PubMed] [Google Scholar]
- Weber ET, Andrade R (2010). Htr2a Gene and 5-HT(2A) Receptor Expression in the Cerebral Cortex Studied Using Genetically Modified Mice. Front Neurosci 4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA (2006). Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313: 536–540 [DOI] [PubMed] [Google Scholar]
- Whorton MR, Bokoch MP, Rasmussen SGF, Huang B, Zare RN, Kobilka B, Sunahara RK (2007). A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proceedings of the National Academy of Sciences of the United States of America 104: 7682–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton MR, Jastrzebska B, Park PS, Fotiadis D, Engel A, Palczewski K, Sunahara RK (2008). Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J Biol Chem 283: 4387–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL (1997). Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse 27: 79–82 [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY (1997). Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther 282: 699–706 [PubMed] [Google Scholar]
- Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN (2008). The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 196: 431–440 [DOI] [PubMed] [Google Scholar]
- Yadav PN, Kroeze WK, Farrell MS, Roth BL (2011). Antagonist functional selectivity: 5-HT2A serotonin receptor antagonists differentially regulate 5-HT2A receptor protein level in vivo. J Pharmacol Exp Ther 339: 99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, Nakao K, Katsuki M, Nakanishi S (1996). Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science 273: 645–647 [DOI] [PubMed] [Google Scholar]
- Young JW, Geyer MA, Rissling AJ, Sharp RF, Eyler LT, Asgaard GL, Light GA (2013). Reverse translation of the rodent 5C-CPT reveals that the impaired attention of people with schizophrenia is similar to scopolamine-induced deficits in mice. Transl Psychiatry 3: e324. [DOI] [PMC free article] [PubMed] [Google Scholar]