Abstract
Purpose:
This study aimed to determine whether PROMIS Physical Function and Pain Interference scores varied at presentation for specialty care by non-trauma hand condition. The secondary aim was to compare PROMIS scores to a reference standard, the QuickDASH, regarding the magnitude and direction of score differentials between diagnoses.
Methods:
PROMIS Physical Function and Pain Interference scores were analyzed from 1471 consecutive new adult patient clinic visits at a tertiary orthopaedic hand clinic presenting with one of 5 non-trauma hand conditions. A 5-point difference on PROMIS assessments was presumed to be clinically relevant. A random sample of 30 QuickDASH scores from each diagnostic group was evaluated for score differentials between groups. We also measured the correlation between PROMIS and QuickDASH scores.
Results:
Patients with carpal tunnel syndrome and thumb basal joint arthritis reported worse Physical Function and more Pain Interference, while those with Dupuytren contractures and ganglion cysts reported less pain and better function. For both domains, patients with trigger fingers averaged PROMIS scores between the other groups. Similar differences were observed in QuickDASH scores as patients with carpal tunnel syndrome and thumb arthritis reported clinically worse upper extremity function than patients with ganglion cysts and Dupuytren contracture. A strong correlation was seen between QuickDASH scores with both PROMIS Physical Function scores and Pain Interference scores.
Conclusions:
PROMIS is sufficiently able to capture differences in self-reported function and pain interference between patients with different hand conditions. PROMIS Physical Function demonstrates construct validity when evaluated against a reference of the QuickDASH across non-trauma hand conditions.
Keywords: PROMIS, function, pain, QuickDASH, hand
INTRODUCTION
With increasing emphasis on patient reported outcomes in healthcare, the National Institute of Health created a comprehensive health outcomes system that can be utilized across all fields of healthcare1–3. This effort led to the development of the Patient-Reported Outcome Measurement Information System (PROMIS), a computerized adaptive testing (CAT) system that includes modules addressing physical, mental, and social health4–7. It has been validated in multiple patient populations, with the ultimate goal of being applicable across all of healthcare7–11.
In populations with upper extremity conditions, general measures of physical function tend to be less specific and responsive than anatomically-specific measures12–14. For example, the Levine-Katz carpal tunnel questionnaire was more sensitive to change in symptoms and function in patients with carpal tunnel syndrome than more generic questionnaires or even physical function measurements12. The patient-rated wrist evaluation (PRWE) and the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire were both more responsive than the Short-Form 36 in patients with a distal radius fracture13. However, both PROMIS Physical Function and Pain Interference scores have demonstrated strong correlations with legacy instruments as well as comparable responsiveness to change when used in hand and upper extremity patient populations15–20. At the same time, PROMIS modules also reduce respondent burden by requiring less time to complete and fewer questions than legacy measures21–23.
While PROMIS Physical Function has demonstrated utility when researching populations with upper extremity conditions, its ideal use involves clinic-wide administration to all patients with varying conditions. If delivered universally as part of routine care, the scores can quantify changes in patients’ perceived function for treating physicians and scores collected at the point of care can be analyzed later to investigate treatment outcomes while avoiding potential recall bias. In this type of heterogeneous population, there are potential differences in pain interference and functional limitations between diagnoses. At this time, it is unclear if PROMIS scores are sufficiently sensitive to capture these differences. Therefore, this study aimed to test the construct validity of PROMIS Physical Function and Pain Interference scores by examining for score variation according to the symptomatic diagnosis and comparing scores to a reference standard of the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH).
METHODS
This cross-sectional evaluation analyzed 1491 consecutive new patient outpatient clinic visits of adult patients presenting to a tertiary orthopaedic hand clinic between 7/1/2015 and 11/30/2016 with one of five non-trauma hand conditions. The study was approved by our Institutional Review Board with a waiver of written consent for the use of data collected during routine clinical care. Visits coded with ICD-10 codes for one of the following conditions were included: carpal tunnel syndrome, Dupuytren contracture, trigger finger, thumb carpometacarpal arthritis, ganglion cyst (Appendix 1). Ganglion cysts predominately originated from the wrist (carpus n=148, interphalangeal joint n=46, flexor tendon sheath=21).
All patients were given a tablet computer (iPad mini, Apple, Cupertino, CA) preloaded with the PROMIS Physical Function (v1.2) and Pain Interference (v1.1) CATs (Computer Adaptive Tests) at check-in. Upon completion, PROMIS scores automatically loaded into the patients’ electronic health record. Visits with at least one valid PROMIS score were included in analysis (n=1471, 98.7%) (Figure 1).
Figure 1.
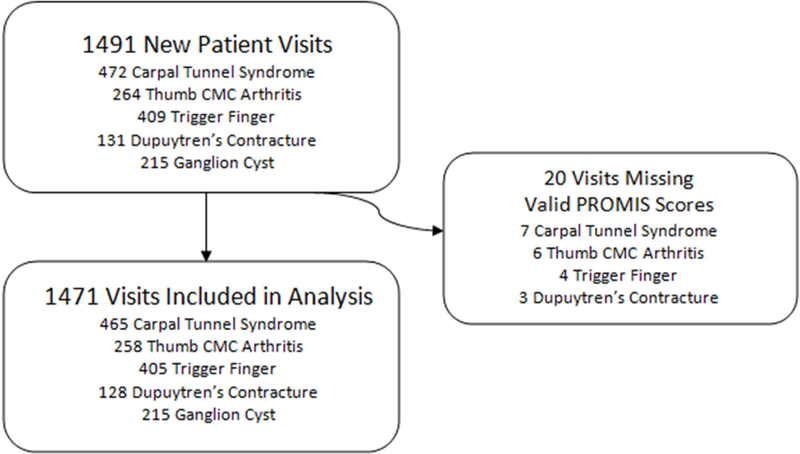
Flowchart of inclusion criteria.
PROMIS assessments were developed by using item response theory (IRT) to narrow a candidate item bank that was tested in the general population24. Questions are then pulled from this item bank in a computerized adaptive testing (CAT) format so that each patient will answer 4–12 questions. All PROMIS modules are normalized to a population mean score of 50 with a standard deviation of 10 points where a higher score represents more of that construct (i.e. a higher Physical Function score represents more, or better, functionality, while a higher Pain Interference score represents more, or worse, pain interference)24. A minimal clinically important difference (MCID) for change in clinical status using PROMIS scores of nearly 5 points has been suggested in other patient populations but not specifically in the conditions studied here 7,25,26. We approached this study considering this 5-point change (Effect Size 0.5) as a reasonable proxy for a clinically relevant difference between groups.
All new patients also completed the paper version of the QuickDASH as a part of their registration packet. The QuickDASH was developed from the Disabilities of the Arm, Shoulder, and Hand Questionnaire27. The QuickDASH is a validated outcome measure which consists of 11 questions, each of which has five Likert-scale responses where 1 indicates the least and 5 the worst function or pain. A difference of 14 points has been determined to be clinically relevant28.
One-way ANOVA with Tukey post-hoc analysis was utilized to assess for age differences between diagnostic groups, while a chi-square analysis with Bonferroni correction assessed differences in sex and race between diagnostic groups.
Identical ANOVA testing compared the effect of the independent variable diagnostic group on PROMIS Physical Function and Pain Interference scores.
To account for patient demographic factors, a generalized linear model (ANCOVA) assessed for differences in marginal means of PROMIS scores between diagnostic groups. Modeling accounted for race and sex (categorical factors) as well as age (continuous covariate). This modeling was performed for both PROMIS Physical Function and Pain-Interference.
As QuickDASH scores were only collected from a subgroup of the study participants, we confirmed the necessary sample size a priori for a one-way ANOVA to determine how many Quick-DASH scores would be required to obtain a power of 0.9 and a two-sided alpha of 0.05 to look for an effect size of 0.4 in the setting of five groups and an assumed standard deviation of 2418,28. This resulted in a total of 100 patients. To protect against increased data variance, scores from 150 patients (30 per group) were analyzed. Systematic random sampling was performed within each diagnostic group to select 30 patients per group. Patients were randomly selected and then charts were manually reviewed for scorable QuickDASH questionnaires. One researcher collected QuickDASH data with a second researcher verifying a random subset of 60 patients to ensure data accuracy.
Nonparametric Kruskal-Wallis Chi-square analyses with post-hoc comparisons using the Mann-Whitney U tested for differences in QuickDASH scores between diagnostic groups as descriptive and graphical examination of the data showed a non-normal distribution. Spearman’s correlation tested the association between QuickDASH scores and PROMIS Physical Function and Pain Interference scores. Correlation coefficients were interpreted as follows: 0.00–0.19 very weak, 0.20–0.39 weak, 0.40–0.59 moderate, 0.60–0.79 strong, 0.80–1.00 very strong29.
RESULTS
Data from 1471 patient visits were included for analysis (Table 1). Patients with carpal tunnel syndrome and ganglion cysts were younger on average than other patients. Dupuytren patients and those with thumb base arthritis were more likely to be Caucasian than other patients, and Dupuytren patients were predominantly male.
Table 1.
Demographic information of study population.
| Overall n=1471 |
CTS n=465 |
Thumb CMC OA n=258 |
Trigger Finger n=405 |
Dupuytren’s n=128 |
Ganglion Cyst n=215 |
|
|---|---|---|---|---|---|---|
| Mean Age (SD) | 57.2 (14.3) | 55.6*(14.7) | 60.8 (9.3) | 60.8 (12.6) | 61.9 (11.6) | 46.9*(17.0) |
| Females (%) | 63.0% | 65.6% | 66.3% | 63.7% | 33.4% | 69.3% |
| Caucasian (%) | 85.2% | 79.5% | 96.0%* | 79.7% | 100.0% | 85.8% |
PROMIS Physical Function and Pain Interference scores varied significantly (p<0.05 and clinically relevant >5 points) between diagnostic groups (Table 2). Physical Function and Pain Interference scores were worst among patients with carpal tunnel syndrome and thumb arthritis, while the least pain and best functionality were reported by those with ganglion cysts or Dupuytren contracture.
Table 2.
PROMIS scores according to diagnosis†
| Physical Function (SD) | Pain Interference (SD) | |
|---|---|---|
| CTS (n=465) | 43.1 a (9.3) | 61.2 a (7.5) |
| Thumb CMC OA (n=258) | 45.9 b (8.1) | 60.0 a,b (6.0) |
| Trigger Finger (n=405) | 47.0 b (9.9) | 58.4 b (7.0) |
| Ganglion Cyst (n=215) | 50.3 c (8.5) | 54.6 c (8.2) |
| Dupuytren’s (n=128) | 51.1 c (10.0) | 52.2 d (9.8) |
Homogenous subsets (p>0.05) represented by alphabetic subscripts
After accounting for demographic characteristics (i.e., age, sex, race), differences in PROMIS scores persisted (Figure 2, Appendices 2–3). Patients with carpal tunnel syndrome and thumb arthritis reported worse physical function than all other groups, while those with Dupuytren contracture and ganglion cysts reported the least pain. In this analysis, race and age each remained significant variables that were associated with mean PROMIS Physical Function and Pain Interference scores. Advancing age and African-American race were associated with worse reported function and greater reported pain.
Figure 2.
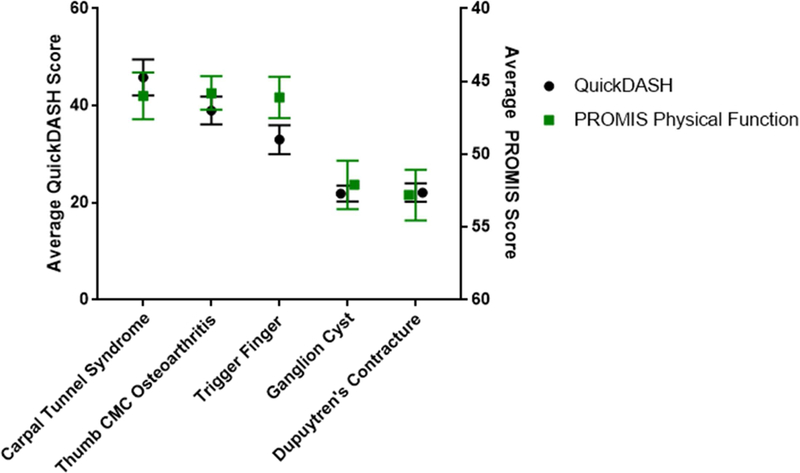
Average QuickDASH and PROMIS Physical Function scores with 95% confidence intervals by diagnosis corrected for race, sex, age.
Similar differences persisted in QuickDASH scores as patients with carpal tunnel syndrome and thumb arthritis reported worse upper extremity function than patients with ganglion cysts and Dupuytren contracture (Figure 2).
Overall, QuickDASH scores were strongly correlated with both PROMIS Physical Function scores (r= −0.66) and Pain Interference scores (r= 0.79).
DISCUSSION
While PROMIS Physical Function and Pain Interference modules are not disease-specific, our data support their construct validity. Scores on these assessments varied with the condition treated in a direction and magnitude similar to the QuickDASH. . Patients with carpal tunnel syndrome reported the worst physical function, and those with thumb arthritis or carpal tunnel reported significantly worse pain interference than patients with Dupuytren contracture or ganglion cysts, even when accounting for differences in age, race, and sex.
The relatively good function experienced by patients with Dupuytren contracture as compared to those with thumb base arthritis, carpal tunnel syndrome, or trigger finger is consistent with prior studies30. Among 262 patients with non-trauma hand conditions, patients with hand arthritis reported worse function on the QuickDASH than patients with carpal tunnel syndrome, trigger finger, and Dupuytren contracture, with the Dupuytren population reporting significantly lower scores than all other groups. This contrasts with Sorensen et al’s baseline QuickDASH scores from a group of 102 patients with hand osteoarthritis, nerve compression syndromes, or tendonitis. No statistically or clinically relevant differences in scores were found, however specific diagnoses were not elucidated28.
Using PROMIS to assess patient outcomes offers the advantages of minimizing patient burden, optimizing testing through computer adaptive testing, and delivering immediate scoring in the electronic health record. The literature has previously described correlations between PROMIS and QuickDASH scores. Overbeek et al found a correlation of r= −0.55 between the QuickDASH and PROMIS Physical Function and r=0.74 between QuickDASH and PROMIS Pain Interference when analyzing scores from 93 consecutive patients presenting to an upper extremity clinic18. Other studies have found correlations with the DASH and PROMIS Physical Function ranging from −0.68 to −0.82 in patients with proximal humerus or rotator cuff pathologies21,22. The strength of correlation between PROMIS and QuickDASH scores across studies has consistently indicated a moderate to strong correlation with minor variation based on the diagnosis studied. Adding further evidence of construct validity, our analysis determined that PROMIS Physical Function/Pain Interference and the QuickDASH all produced similar relative rankings of diagnoses according to their health impact.
An upper extremity specific measure of physical function, the Upper Extremity – Physical Function CAT, has been developed for PROMIS. Scores from the Upper Extremity CAT have been shown to correlate as well as those from the PROMIS Physical Function CAT with the QuickDASH and DASH16,17. However, the Upper Extremity CAT has a ceiling effect and currently cannot discriminate between higher levels of function, making it difficult to solely utilize the Upper Extremity CAT as a measure of function16,17,31–33. Notably, the PROMIS Upper Extremity CAT continues to be modified as new versions are released. However, these limitations have persisted, albeit to lesser degrees, with the latest Upper Extremity CAT. We acknowledge that the PROMIS Upper Extremity CAT may eventually supplant the PROMIS Physical Function CAT in hand practices, but until that time our data indicate that the Physical Function CAT can discriminate among hand conditions.
Accounting for diagnosis and sex, patient age and race were significant predictors of PROMIS Physical Function and Pain Interference scores. However, due to the lack of details about symptom severity and acuity, it is impossible to determine whether these differences are due to worse perception of similar symptoms, or whether individuals in this population presented with more severe disease processes. Similarly, the unavailability of disease history as well as the patients’ socioeconomic status makes interpreting differences in average PROMIS scores by racial groups difficult. Although this study was not designed to explain why PROMIS score differences may occur between patients according to demographic data, it was necessary to control for these variables during analysis to account for predictable demographic differences between diagnostic groups (e.g. the predominance of Caucasian males within the Dupuytren population).
Our study has several limitations. The cross-sectional design does not allow longitudinal data collection, which is necessary to determine if the PROMIS measures demonstrate responsiveness to treatment when compared to the QuickDASH. Second, the large number of patients studied allows appreciation of general patterns, but does not account for variability in the acuity, severity, or comorbid diseases in these patients. Finally, we only compared PROMIS scores to the QuickDASH. It is possible that the choice of an alternative reference standard (e.g., Michigan Hand Questionnaire) could have affected our results. However, we have no reason to suspect that the overall patterns of differences would change.
In conclusion, we have found that PROMIS captures differences in self-reported function and pain interference between patients with different hand conditions. Additionally, these differences correlate with those captured by the QuickDASH, a well-validated and extensively cited measure of upper extremity impairment. The consistency of our findings when using PROMIS scores and the QuickDASH suggests that these differences are more likely true differences attributable to specific diagnoses as opposed to a finding specific to a single patient-reported outcome measure. Our data suggest that PROMIS Physical Function is well suited for widespread delivery in the upper extremity practice.
Clinical Relevance Statement:
The use of PROMIS is expanding but as PROMIS is not disease specific, assessment of its construct validity is necessary for hand conditions.
Acknowledgments
Funding: Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448, sub-award TL1TR000449, from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), and Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842, which supported the maintenance and use of REDCap electronic data capture tools, hosted in the Biostatistics Division of Washington University School of Medicine.. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. This funding did not play a direct role in this investigation.
Appendix 1. International Classification of Diseases Codes.
| Carpal Tunnel | G56.00 | G56.11 |
| Syndrome | G56.01 | G56.12 |
| G56.02 | ||
| Thumb CMC Arthritis | M18.0 | M18.31 |
| M18.11 | M18.32 | |
| M18.12 | M18.9 | |
| Trigger Finger | M65.30 | M65.332 |
| M65.311 | M65.339 | |
| M65.312 | M65.341 | |
| M65.319 | M65.342 | |
| M65.321 | M65.349 | |
| M65.322 | M65.351 | |
| M65.329 | M65.352 | |
| M65.331 | M65.359 | |
| Ganglion Cyst | M67.40 | M67.441 |
| M67.431 | M67.442 | |
| M67.432 | M67.449 | |
| M67.439 | ||
| Dupuytren’s Contracture |
M72.0 | |
Appendix 2. Between-subject effect of independent variables on PROMIS Physical Function in ANCOVA analysis.
| Variable | p-value |
|---|---|
| Diagnosis Group | <0.05 |
| Age | <0.05 |
| Sex | 0.14 |
| Race | <0.05 |
Appendix 3. Between-subject effect of independent variables on PROMIS Pain Interference in ANCOVA analysis.
| Variable | p-value |
|---|---|
| Diagnosis Group | <0.05 |
| Age | <0.05 |
| Sex | 0.13 |
| Race | <0.05 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care 2007;45(5 Suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rineberg BA. A call to leadership. The role of orthopaedic surgeons in musculoskeletal outcomes research. J Bone Joint Surg Am 1990;72(10):1439–1440. [PubMed] [Google Scholar]
- 3.Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: A new era in clinical research. Perspect Clin Res 2011;2(4):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fries JF, Witter J, Rose M, Cella D, Khanna D, Morgan-DeWitt E. Item response theory, computerized adaptive testing, and PROMIS: assessment of physical function. J Rheumatol 2014;41(1):153–158. [DOI] [PubMed] [Google Scholar]
- 5.Hung M, Franklin JD, Hon SD, Cheng C, Conrad J, Saltzman CL. Time for a paradigm shift with computerized adaptive testing of general physical function outcomes measurements. Foot Ankle Int 2014;35(1):1–7. [DOI] [PubMed] [Google Scholar]
- 6.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broderick JE, Schneider S, Junghaenel DU, Schwartz JE, Stone AA. Validity and reliability of patient-reported outcomes measurement information system instruments in osteoarthritis. Arthritis Care Res (Hoboken) 2013;65(10):1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cella D, Gershon R, Lai J-S, Choi S. The future of outcomes measurement: item banking, tailored short-forms, and computerized adaptive assessment. Quality of Life Research 2007;16(1):133–141. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett SJ, Orbai AM, Duncan T, et al. Reliability and Validity of Selected PROMIS Measures in People with Rheumatoid Arthritis. PLoS One 2015;10(9):e0138543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn KE, Dew MA, Lin L, et al. Reliability and construct validity of PROMIS® measures for patients with heart failure who undergo heart transplant. Qual Life Res 2015;24(11):2591–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howell CR, Gross HE, Reeve BB, DeWalt DA, Huang IC. Known-groups validity of the Patient-Reported Outcomes Measurement Information System (PROMIS(®)) in adolescents and young adults with special healthcare needs. Qual Life Res 2016;25(7):1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amadio PC, Silverstein MD, Ilstrup DM, Schleck CD, Jensen LM. Outcome assessment for carpal tunnel surgery: the relative responsiveness of generic, arthritis-specific, disease-specific, and physical examination measures. J Hand Surg Am 1996;21(3):338–346. [DOI] [PubMed] [Google Scholar]
- 13.MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the short form-36, disability of the arm, shoulder, and hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am 2000;25(2):330–340. [DOI] [PubMed] [Google Scholar]
- 14.Gay RE, Amadio PC, Johnson JC. Comparative responsiveness of the disabilities of the arm, shoulder, and hand, the carpal tunnel questionnaire, and the SF-36 to clinical change after carpal tunnel release. J Hand Surg Am 2003;28(2):250–254. [DOI] [PubMed] [Google Scholar]
- 15.Brodke D, Saltzman C, Brodke D. PROMIS for Orthopaedic Outcomes Measurement. Journal of the American Academy of Orthopaedic Surgeons 2016;24(11):744–749. [DOI] [PubMed] [Google Scholar]
- 16.Beckmann JT, Hung M, Voss MW, Crum AB, Bounsanga J, Tyser AR. Evaluation of the Patient-Reported Outcomes Measurement Information System Upper Extremity Computer Adaptive Test. J Hand Surg Am 2016;41(7):739–744.e734. [DOI] [PubMed] [Google Scholar]
- 17.Döring AC, Nota SP, Hageman MG, Ring DC. Measurement of upper extremity disability using the Patient-Reported Outcomes Measurement Information System. J Hand Surg Am 2014;39(6):1160–1165. [DOI] [PubMed] [Google Scholar]
- 18.Overbeek CL, Nota SP, Jayakumar P, Hageman MG, Ring D. The PROMIS physical function correlates with the QuickDASH in patients with upper extremity illness. Clin Orthop Relat Res 2015;473(1):311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyser AR, Beckmann J, Franklin JD, et al. Evaluation of the PROMIS physical function computer adaptive test in the upper extremity. J Hand Surg Am 2014;39(10):2047–2051.e2044. [DOI] [PubMed] [Google Scholar]
- 20.Fries J, Krishnan E, Rose M, Lingala B, Bruce B. Improved Responsiveness and Reduced Sample Size Requirements of PROMIS Physical Function Scales with Item Response Theory. Arthritis Research and Therapy 2011;13(5):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan JH, Kallen MA, Okike K, Lee OC, Vrahas MS. PROMIS Physical Function Computer Adaptive Test Compared With Other Upper Extremity Outcome Measures in the Evaluation of Proximal Humerus Fractures in Patients Older Than 60 Years. J Orthop Trauma 2015;29(6):257–263. [DOI] [PubMed] [Google Scholar]
- 22.Beckmann JT, Hung M, Bounsanga J, Wylie JD, Granger EK, Tashjian RZ. Psychometric evaluation of the PROMIS Physical Function Computerized Adaptive Test in comparison to the American Shoulder and Elbow Surgeons score and Simple Shoulder Test in patients with rotator cuff disease. J Shoulder Elbow Surg 2015;24(12):1961–1967. [DOI] [PubMed] [Google Scholar]
- 23.Senders A, Hanes D, Bourdette D, Whitham R, Shinto L. Reducing survey burden: feasibility and validity of PROMIS measures in multiple sclerosis. Mult Scler 2014;20(8):1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain 2010;150(1):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amtmann D, Kim J, Chung H, Askew RL, Park R, Cook KF. Minimally important differences for Patient Reported Outcomes Measurement Information System pain interference for individuals with back pain. J Pain Res 2016;9:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol 2011;64(5):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beaton DE, Wright JG, Katz JN, Group UEC. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am 2005;87(5):1038–1046. [DOI] [PubMed] [Google Scholar]
- 28.Sorensen AA, Howard D, Tan WH, Ketchersid J, Calfee RP. Minimal clinically important differences of 3 patient-rated outcomes instruments. J Hand Surg Am 2013;38(4):641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans JD. Straightforward statistics for the behavioral science Pacific Grove, CA: Brooks/Cole Publishing; 1996. [Google Scholar]
- 30.Van Vliet MM, Maradey JA, Homa KA, Kerrigan CL. The usefulness of patient-reported measures for clinical practice. Plast Reconstr Surg 2013;132(1):105–112. [DOI] [PubMed] [Google Scholar]
- 31.Hung M, Voss MW, Bounsanga J, Crum AB, Tyser AR. Examination of the PROMIS upper extremity item bank. J Hand Ther 2016. [DOI] [PMC free article] [PubMed]
- 32.Anthony CA, Glass NA, Hancock K, Bollier M, Wolf BR, Hettrich CM. Performance of PROMIS Instruments in Patients With Shoulder Instability. Am J Sports Med 2017;45(2):449–453. [DOI] [PubMed] [Google Scholar]
- 33.Beleckas CM, Padovano A, Guattery J, Chamberlain AM, Keener JD, Calfee RP. Performance of Patient-Reported Outcomes Measurement Information System (PROMIS) Upper Extremity (UE) Versus Physical Function (PF) Computer Adaptive Tests (CATs) in Upper Extremity Clinics. J Hand Surg Am 2017;42(11):867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]


