Abstract
In the ascidian Ciona intestinalis, oral siphon amputation activates adult stem cell niches in the branchial sac to divide and dispatch migratory progenitor cells to a regeneration blastema at the site of injury. This study shows that progenitor cells derived from branchial sac stem cell niches have roles in homeostasis, wound repair, and regeneration of the siphons and neural complex (NC). During homeostasis, progenitor cells targeted the pharyngeal stigmata to replace ciliated cells involved in filter feeding. After individual or double siphon amputations, progenitor cells specifically targeted the oral or atrial siphons or both siphons, and were involved in the replacement of siphon circular muscle fibers. After oral siphon wounding, progenitor cells targeted the wound sites, and in some cases a supernumerary siphon was formed, although progenitor cell targeting did not predict the induction of supernumerary siphons. Following NC ablation, progenitor cells specifically targeted the regenerating NC, and supplied the precursors of new brain and neural gland cells. The tissues and organs targeted by branchial sac stem cells exhibited apoptosis during homeostasis and injury. It is concluded that branchial sac progenitor cells are multipotent and show targeting specificity that is correlated with apoptosis during homeostatic growth, tissue repair, and regeneration.
Keywords: Ciona intestinalis, branchial sac stem cell niche, progenitor cells, migratory specificity, homeostasis, wound repair, regeneration, apoptosis
Introduction
An important property of adult stem cells and the migratory progenitor cells they produce is their ability to accurately target tissues and organs for effective replacement during homeostatic growth and injury. Although progress has been made in this area during the last decade (Lucas et al., 2018), knowledge about how stem cells respond to signals by directing the migration of progenitor cells to their appropriate places is still incomplete. Progenitor cell targeting is most effectively studied in organisms with high capacities for regeneration. The tunicates are known to show extensive regenerative capacities (Berrill, 1951; Candia Carnevali and Burighel, 2010). The colonial ascidian tunicates, such as members of the genera Botryllus and Botrylloides, Clavelina, and Perophora, can replace their entire bodies from small parts of the basal vasculature (whole body regeneration), the mid-section of a zooid, or initiate regeneration using individual blood cells, respectively (Tiozzo et al., 2008; Brown et al., 2009; Brien, 1968; Freeman, 1964). Whole body regeneration in colonial ascidians involves the activity of hemocyte-like stem cells that are located in the vasculature and/or the endostyle (Rinkevich et al., 2010; Voskoboynik et al., 2008). Ascidians can also regenerate the entire central nervous system (Schultze, 1899; Bollner, 1989; Dahlberg et al., 2009), a phenomenon that is unique among the chordates.
Ciona (C. intestinalis and C. robusta) is the solitary ascidian most frequently used as a model in regeneration research (Dahlberg et al., 2009; Jeffery, 2015a; 2015b; 2015c; Hamada et al., 2015; Spina et al., 2017). The many attractive aspects of Ciona for regeneration studies are highlighted by a large and transparent body and the ability to survive many types of surgical operations. However, Ciona and other solitary ascidians have more limited regenerative capacities than colonial ascidians: proximal parts of the body can replace distal parts, such as the siphon and central nervous system, but distal parts are unable to regenerate proximal parts, such as the heart, gonad, and viscera (Jeffery, 2015c). The limited and axially polarized regenerative potential of the solitary ascidians is termed partial or distal body regeneration (Jeffery, 2015b). Interestingly, distal body regeneration is dependent on the inclusion of at least a part of the branchial sac in the regenerating proximal sector (Hirschler, 1914; Jeffery, 2015b). The branchial sac is a large feeding and respiratory organ punctuated by perforations called stigmata (Manni et al., 2002; Shimazaki et al., 2006). The stigmata are lined by ciliated cells that drive water flow through the pharynx between the oral and atrial siphons during filter feeding. As Ciona grows, the branchial sac increases in size, and the stigmata become larger and replicate by longitudinal fission (Millar, 1953).
Ciona can rapidly and precisely regenerate the entire oral siphon (Whittaker, 1975; Auger et al., 2010; Jeffery 2015c). Several different experiments have implicated progenitor cells produced by PIWI (an Argonaute family protein involved in maintaining stemness) and alkaline phosphatase (AP) positive stem cells located in the transverse vasculature of the branchial sac in oral siphon regeneration (Jeffery, 2015b). First, oral siphon amputation followed by a short pulse of 5-ethynyl-2´-deoxyuridine (EdU) preferentially labels dividing stem cells in the branchial sac vasculature. Second, an EdU pulse-chase results in the appearance of EdU labeled progenitor cells in the oral siphon blastema. Third, transplantation of branchial sac explants from EdU labeled donors into unlabeled hosts shows that labeled progenitor cells derived from the grafts target the regenerating host oral siphons. Once the progenitor cells migrate and form the regeneration blastema, they up-regulate a suite of genes, including members of the delta-notch signaling pathway, molecules involved in tissue repair, and cell guidance factors (Hamada et al., 2015). The progenitor cells of the blastema probably interact with the epidermal and endodermal epithelia at the amputation site to produce a new oral siphon. The possible roles of the branchial sac stem cell niches in the regeneration of other distal organs, in tissue repair after wounding, and in homeostasis have not been studied.
The ascidian central nervous system or neural complex (NC) consists of the cerebral ganglion (brain), the source of efferent nerve tracts that radiate anteriorly and posteriorly throughout the body (Osugi et al., 2017), and the underlying neural gland, an organ of uncertain function (Deyts et al., 2006). Dahlberg et al (2009) have described the following sequence of events in Ciona NC regeneration: wound healing at the ablation site, the gradual extension of severed nerve tracts into the wounded area, neurogenesis and reorganization of NC structure, and restoration of NC physiological functions. The latter investigation also reported a blastema of proliferating cells at the site of NC regeneration, but the identity and source of these cells was unresolved. Three hypotheses have been offered to explain the source of progenitor cells for NC regeneration: (1) the migration of neuroblasts derived from the dorsal strand nerve plexus lying posterior to the NC (Fedele, 1938; Bollner et al., 1997), (2) the invasion of neural precursors from the severed nerve tracts surrounding the ablated NC (Dahlberg et al., 2009), and (3) the accumulation of inflammatory hemocytes derived from the circulatory system (Medina et al., 2014).
The purpose of the present study was to investigate the multiple roles and targeting specificity of branchial sac progenitor cells during homoeostatic growth, injury, and siphon and NC regeneration in Ciona intestinalis. Using the standard EdU pulse-chase labeling approach (Jeffery, 2015b), it is shown that the branchial sac stigmata, distal wounds, siphons, and NC all use branchial sac progenitor cells for tissue renewal or replacement during homeostasis, tissue repair, and regeneration, respectively. This study also reveals the specificity of progenitor cell migration and suggests a possible role for apoptosis in guiding the progenitor cells to their appropriate targets.
Materials and Methods
Biological materials
Ciona intestinalis was collected from harbors near Woods Hole, MA, from natural running seawater aquaria in the Marine Resources Center, Marine Biological Laboratory, Woods Hole, MA, or cultured from fertilized eggs grown on Petri dishes in the laboratory as described by Jeffery (2015b). The animals used in these experiments ranged from about 2–8 cm in length and were aged from 2–6 months.
Surgical procedures
Animals were anesthetized by submersion for about 15 min at room temperature in 1 mg/ml tricaine methanesulphonate (MS222; Sigma-Aldrich, St. Louis, MO) dissolved in seawater. Surgical operations were carried out using sharp dissection scissors or straight-bladed fine spring scissors (5 or 8 mm; Fine Science Tools, Inc, Foster City, CA) and fine forceps. Oral siphons were amputated just above the level of the tentacles along a plane perpendicular to the proximal–distal axis. Atrial siphons were amputated through a perpendicular plane near the base. In some animals, both the oral and atrial siphons were amputated in immediate succession using the procedures described above. To produce wounds, the oral siphon wall was punctured or cut horizontally through the tunic. To ablate the NC, a small hole was made in the tunic between the oral and atrial siphons, and the NC was severed from connecting nerve tracts and excised through the hole. In sham-operated controls, the NC was exposed as described above and scrambled with a dissecting pin but not removed. The operated and control animals were maintained in aquaria with running sea water at ambient temperature.
Alkaline phosphatase staining
Stem cell staining with alkaline phosphatase (AP) was carried out with animals removed from their tunics as described by Jeffery (2015b). The naked animals were fixed in 4% paraformaldehyde (PFA) in Millipore filtered sea water (MFSW) for 1 hr at room temperature, washed three times in PBS, and then treated with NBI/BCIP (Invitrogen, Carlsbad, CA.) at room temperature in the dark. The progress of staining was monitored by microscopy, and the animals were washed three times in PBS and imaged after blue color developed in the transverse vessels of the branchial sac.
Phalloidin staining
For staining of actin in muscle fibers, animals were fixed in 5% formalin in MFSW for 1 hr at room temperature, washed three times in PBS (10 min), incubated in PBS containing 0.1% Triton X-100 (30 min), washed three times in PBS (10 min), and incubated with 25μg/ml of rhodamine-phalloidin (Molecular Probes, Eugene, OR) at room temperature in the dark. After staining, the specimens were washed three times in PBS (10 min), and imaged by fluorescence microscopy.
EdU pulse-chase labeling
Operated and control animals were subjected to a standard pulse-chase labeling protocol with 5-ethynyl-2’-deoxyurdine (EdU; Invitrogen) to label dividing progenitor cells in the branchial sac (Jeffery, 2015b). This protocol involved a pulse labeling step, done by incubating animals with 200 μM EdU (Invitrogen) in MFSW for 24 hrs, and a subsequent chase step, which was carried out by rinsing animals five times successively with 1000 ml volumes MFSW, and then placing animals in a large aquarium with running sea water for the duration of the chase. The chase was conducted for 2–8 days for siphon amputated or wounded animals and controls and for 4–40 days for NC ablated animals and sham operated controls. After the completion of the chase, the operated animals and controls were fixed for 14 hrs in 4% PFA, rinsed 3 times in 1 X PBS, treated with 0.5% Triton X-100 in PBS for 1 hr, rinsed 3 times in PBS, and processed for EdU detection by staining with Alexa Fluor azide 488 or 594 at room temperature according to the directions supplied with the Click-iT™ EdU Alexa Fluor High Throughput Imaging Assay Kit (Invitrogen).
The EdU incorporated specimens were imaged by fluorescence microscopy either as whole animals or flat mounts prepared as described by Auger et al. (2010). EdU labeling was quantified as described in the figure legends. Some EdU labeled specimens were post fixed in 4% PFA in PBS overnight at 4°C, embedded in Paraplast (Polysciences, Warrington, PA), sectioned at 10 μm, attached to glass slides, and imaged by fluorescence microscopy.
TUNEL staining
Apoptosis was detected by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Normal and operated animals were anesthetized, their tunics were removed as described above, and they were fixed in 4% PFA in PBS overnight at 4°C. After fixation, the specimens were washed twice for 5 min in 1 X PBS; 0.1% Triton X-100 (PBST) at room temperature and subjected to one of the following procedures. First, to detect apoptosis during homeostatic growth and after NC removal, the fixed animals were severed across their midsections into two parts to expose the internal organs, and the body parts were further processed separately as described below. Second, to detect apoptosis after siphon wounding or amputation, the siphon was severed from the body and processed as described below. The body parts were suspended in proteinase K (10 μg/ml) for 15 min 37°C, and then washed with PBST. The TUNEL assay was performed using the In Situ Cell Death Kit (Roche) according to the instructions supplied by the manufacturer. The processed specimens were post-fixed in 4% PFA in PBS overnight at 4°C, embedded in Paraplast, sectioned at 10 μm, the sections were attached to gelatin subbed glass slides, and the unstained sections were imaged by microscopy.
Tubulin Immunostaining
Detection of ciliated stigmatal cells in the branchial sac was done by immunostaining with alpha-tubulin antibody (Invitrogen) as described by Jeffery et al. (2008). Briefly, animals were fixed in 4% PFA in PBS (pH 7.0; overnight), stained with primary antibody diluted 1:100 in PBS, and antigen−antibody complexes were detected with biotinylated anti-rabbit IgG secondary antibody (1:200 in PBS; Vector Laboratories, Burlingame, CA, USA) using the ABC Peroxidase Kit (Vector Laboratories). The stained specimens were post-fixed with 4% PFA in PBS overnight at 4°C, embedded in polyester wax, sectioned, mounted on glass slides as described above, and imaged by microscopy.
Results
Branchial sac progenitor cells target the stigmata during homeostasis
PIWI and AP positive stem cell niches are located in the transverse vessels of the Ciona branchial sac (Fig. 1A, B). The transverse and longitudinal vessels form a meshwork surrounding each branchial sac fissure or stigmata (Fig. 1B; Berrill, 1947; Chiba et al., 2004). EdU pulse-chase experiments have shown that stem cells located in the transverse vessels provide the progenitor cells for distal regeneration (Jeffery, 2015b).
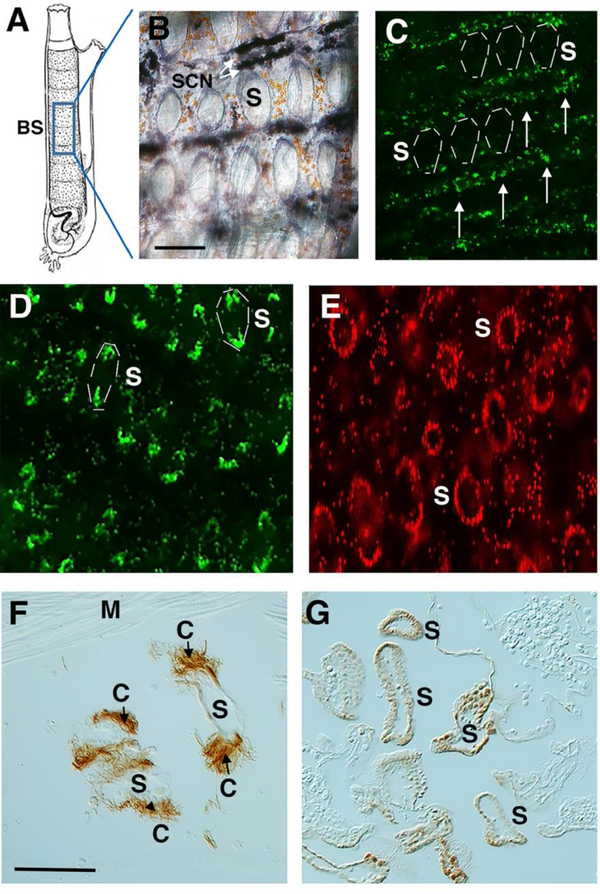
Figure 1.
Branchial sac progenitor cells target pharyngeal stigmata during homeostasis. A. A diagram of an adult Ciona showing the location in the branchial sac (BS) in images B-E. B. A branchial sac stained with alkaline phosphatase (AP) showing stem cell niches (SCN, arrows) lining the transverse vessels. S: stigmata. C. Fluorescence image of the branchial sac from an animal exposed to EdU for 24 hrs showing labeling in progenitor cells (arrows) associated with the stem cell niche. A few stigmata (S) are indicated by dashed lines. D. Fluorescence image of the branchial sac of an animal subjected to EdU for 24 hrs followed by a 2-day chase showing labeling of cells at the distal and proximal edges of stigmata (S). A few labeled stigmata are indicated by dashed lines. E. Fluorescence image of the branchial sac of an animal subjected to EdU for 24 hrs followed by a 4-day chase showing labeled cells around the circumference of stigmata (S). Scale bar: 20 μm in B-E. F. Section through a branchial sac stained with tubulin antibody showing ciliated cells (C) in stigamta (S). M: unstained muscle fibers. G. Apoptosis of stigmatal cells. A section through a branchial sac showing TUNEL labeled cells bordering the stigmatal fissures (S). Scale bars: 20 μm in F, G.
To determine whether the branchial sac progenitor cells have roles in normal growth, uninjured animals were subjected to the standard EdU labeling period of 24 hrs followed by a 2–4-day chase (Jeffery, 2015b), and EdU incorporation in the branchial sac was determined (Fig. 1C-E). During the EdU pulse, progenitor cells in the transverse vessels were labeled, whereas the reminder of the branchial sac, including cells lining the stigmata, were unlabeled (Fig. 1C; also see Fig. 2B). After 2 days of chase, EdU labeling was reduced in the transverse vessels and appeared in cells located in the distal and proximal margins of the pharyngeal stigmata (Fig. 1D), which are likely to represent stigmatal growth zones. By the end of 4 days of chase, EdU labeled cells were seen throughout the circumference of the pharyngeal stigmata (Fig. 1E). The results show that progenitor cells dispatched from branchial sac stem cell niches target the pharyngeal stigmata during homeostasis.
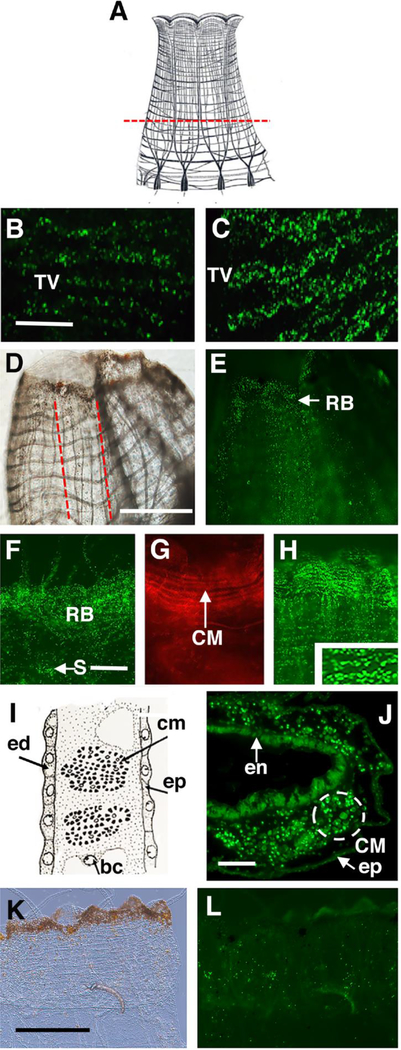
Figure 2.
Oral siphon amputation activates cell division in the branchial sac, migration of progenitor cells to the blastema, and progenitor cell differentiation into circular muscle fibers. A. A diagram of the oral siphon showing the position of the amputation plane (dashed line). B. C. EdU incorporation into the transverse vessels (TV) of the branchial sac of a control (B) and an oral siphon amputated animal (C) after a 24 hr EdU pulse. Scale bar: 20 μm in B, C. D, E. EdU labeled cells in longitudinal columns (outlined by red dashed lines in D) and the regeneration blastema (RB) of an oral siphon following a 2-day chase. D and E show bright field and fluorescence images of the same regenerating siphon. Scale bar: 400 μm in D, E. F. EdU labeling is scattered through cells in the regeneration blastema (RB) and in stigmata (S) of the branchial sac following a 24 hr pulse and 4 days of chase. G. Rhodamine-phalloidin labeling of regenerating circular muscle bands after 8 days of chase. H. EdU labeling is associated with horizontal lines of circular muscle fibers (CM) (inset 2X magnification of boxed area) after a 24 hr pulse followed by 8 days of chase. Scale bar: 40 μm in F-H. I. Diagram of a sectioned oral siphon body wall showing epidermis (ep), endodermis (ed), a blood cell (bc), and bundles of circular muscle fibers (cm). After Millar (1953). J. Section of a regenerating oral siphon through a horizontal plane showing bundles (dashed circle) of EdU labeled circular muscle cells (CM). Scale bar: 25 μm. K, L. Low levels of EdU incorporation in the oral siphon of a control following a 24 hr pulse and 8 days of chase. K and L show bright field (K) and fluorescence (L) images of the same siphon. Scale bar: 500 μm.
The duration of the pulse-chase experiments described above was 6 days, and the appearance of EdU labeled cells around the circumference of the stigmata during this interval suggests a high level of cell turnover and replacement. Tissues subject to high turnover can exhibit correspondingly high levels of apoptosis, as older cells die, are removed, and replaced with new copies. To address the question of apoptosis, uninjured animals were subjected to the TUNEL assay, and then sectioned to assess TUNEL-labeling in the branchial sac (Fig 1F, G). Staining with anti-tubulin confirmed the identity of the stigmatal cells, which exhibited tufts of ciliary microtubules (Fig. 1F). Sections of TUNEL stained animals showed stigmata with many apoptotic cells (Fig. 1G), whereas the surrounding tissues, including the transverse and longitudinal vessels of the branchial sac, the endostyle, and body wall muscle bands were not stained by TUNEL. The results show that homeostatic growth involves apoptosis of the stigmatal cells and their replacement by progenitors derived from the branchial sac stem cell niche.
Branchial sac progenitor cells target the blastema to form circular muscle fibers during oral siphon regeneration
To determine the fate of progenitor cells from the branchial sac during distal regeneration, the oral siphon was amputated (Fig. 2A) and the amputated animals and uninjured controls were exposed to the standard EdU labeling period followed by 2–8 days of chase. The amputated animals showed higher levels of EdU labeling in transverse vessels of the branchial sac than controls (Fig. 2B, C), but low levels of labeling we seen in other regions of the body, including the amputation sites (Jeffery, 2015b). During the chases, EdU labeled progenitor cells accumulated in the oral siphons of amputated (Fig. 2D-H, J) but not control animals (Fig. 2K, L). Furthermore, as seen in uninjured animals during homeostasis (Fig. 1), EdU labeled cells were also chased into the branchial sac stigmata (Fig. 2F). After 2 days of chase, EdU labeled cells were detected in wide longitudinal zones in the siphon stump (Fig. 2D, E), which probably represent vascular sinuses through which progenitor cells migrate and eventually populate the distal edge of the early regenerating siphon. After 4 days of chase, EdU labeled cells were distributed randomly in a blastema in the regenerating oral siphon (Fig 2F) (Auger et al, 2010; Jeffery, 2015b). After 8 days of chase, however, many of the EdU labeled cells became organized along horizontal lines (Fig. 1H), suggesting an association with bundles of regenerating circular muscle fibers (Fig. 1G), which form in the blastema perpendicular to the long axis of the regenerating siphon (Hamada et al., 2015). Sections cut perpendicular to the proximal-distal cylindrical axis of the regenerating siphon after 8 days of chase confirmed EdU labeling in bundles of circular muscle fibers (Fig 2I, J). The results suggest that progenitor cells dispatched from the branchial sac form a regeneration blastema and contribute to the newly formed circular muscle fibers during oral siphon regeneration.
Targeting specificity of progenitor cells during siphon regeneration
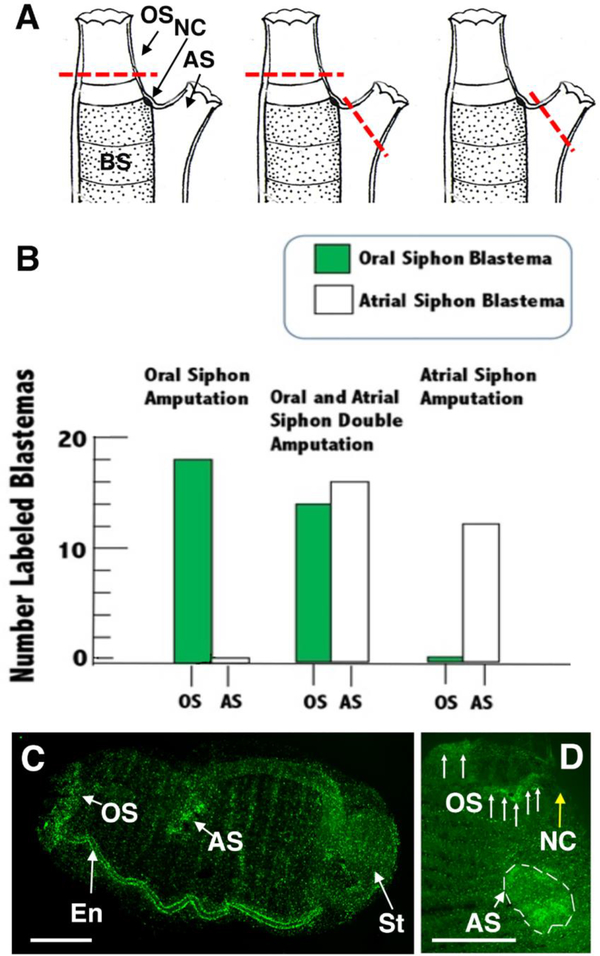
The specificity of progenitor cells in targeting specific regenerating siphons was determined by comparing the effects of amputating the oral siphon, the atrial siphon, or both the oral and atrial siphons (Fig. 3A) after the standard EdU pulse-chase protocol was applied to identify progenitor cells derived from the branchial sac. As described above (Fig. 2), oral siphon amputation resulted in the formation of a blastema of EdU labeled progenitor cells in the regenerating oral siphon, but not in the un-amputated atrial siphon (Fig. 3B left). In contrast, when the atrial siphon was excised, an EdU labeled blastema was formed in the atrial siphon, but not in the oral siphon (Fig. 3B, right). Finally, when both the oral and atrial siphons were amputated, EdU labeled blastemas were formed in both regenerating siphons (Fig. 3B middle, C, D). It was previously reported that the endostyle was sometimes labeled during the chase but not in the EdU pulse after oral siphon removal (Jeffery, 2015b). In the present study, endostyle labeling was seen occasionally during the chase following oral (2 of 18 cases) or atrial siphon (1 of 12 cases) amputation, but observed more frequently (10 of 16 cases) after both siphons were removed and were regenerating (Fig. 3C), implying that progenitor cells can be detected more frequently in double siphon amputations. The results suggest that progenitor cell targeting is specific for the sites of siphon regeneration, and that these cells may transit from the branchial sac stem cell niches to distal locations in the body via the endostyle.
Figure 3.
Specificity of progenitor targeting and blastema formation during regeneration of the oral, atrial, or both siphons. A. Diagrams showing the positions of amputation (red dashed lines) in different types of siphon regeneration experiments. Left. oral siphon (OS) amputation. Middle. Oral and atrial siphon (AS) double amputation. Right. Atrial siphon amputation. BS: branchial sac. NC: neural complex. B. Bar graphs showing the numbers of oral and atrial siphons with EdU labeled blastemas after oral (left), oral and atrial (middle), or atrial (right) siphon amputation of the same animals. N of animals with amputated siphons: 18 (oral siphon), 16 (both siphons), and 12 (atrial siphon). C, D. EdU labeled blastema formation following amputation and regeneration of both oral and atrial siphons following a 24 hr EdU pulse and a 2 (C) or 4(D) day chase. C. Fluorescence image showing EdU labeled oral siphon (OS) and atrial siphon (AS) blastemas and labeled endostyle (En) in an entire regenerating animal. The stomach (St) is also labeled (see Jeffery, 2015b). Scale bar: 60 μm. D. Fluorescence image of the distal region of another double amputated animal regenerating both siphons. Arrows indicate parts of oral siphon (OS) blastema and dashed lines indicate atrial siphon (AS) blastema. Scale bar: 40 μm. NC (yellow arrow): neural complex.
Progenitor cell targeting during wounding and the induction of supernumerary siphons
To determine whether progenitor cell migration from the branchial sac can be induced by distal injuries other than siphon amputation, we applied the standard pulse-chase EdU labeling protocol to animals with wounded oral siphons (Fig 4A, B). Small or large wounds in the siphon wall were created without removing any part of the siphon itself (Fig. 4A-D). After about a day the wounded areas became opaque due to the accumulation of yellow pigment cells (see Fig. 6C) in the otherwise transparent siphon. After the end of the EdU labeling period no labeled cells were seen near the wounds (Fig. 4E, F), although stem cells were activated to divide in the branchial sac, as occurred after siphon amputations (Figs. 2, 3). After 2 days of chase, some wounds had been repaired and sealed (Fig. 4K, L), whereas others, both small (Fig. 4C) and large (Fig. 4D), were still open, and orange pigment organs (OPO) had appeared on their proximal edges.
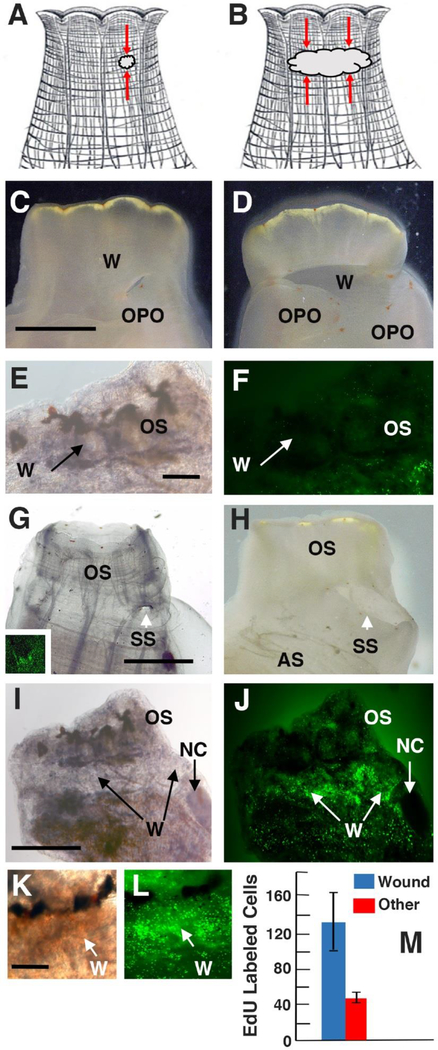
Figure 4.
Branchial sac progenitor cells target distal wounds and participate in the formation of supernumerary siphons. A, B. Diagrams showing the positions (red arrows) of small (A) and large (B) wounds in oral siphons. C, D. Oral siphons with small (C) or large (D) open wounds 6 days after wounding showing orange pigment organs (OPO) at the proximal margin of the wounds (W). Scale bar: 3 mm in C, D. E, F. A wounded oral siphon (OS) at the end of the 24 hr EdU pulse showing no labeling in the wounded area (W). Scale bar: 400 μm in E, F. G, H. Oral siphons with small (G) or large (H) wounds (W) 14 days after injury showing the formation of small (G) or large (H) supernumerary siphons (SS). Scale bar: 1 mm in G, H. In the following experiments, siphon wounding was followed by a 24 hr EdU pulse and a 4-day chase. Inset of G, I-L. EdU labeling following the chase surrounding a small wound (inset of G), a large (I, J) open wound, and (K, L) a large sealed wound (W). Bright field images: C-E, G, I, K). Fluorescence images (F, inset of G, J, L). Scale bar: 600 μm in I, J, and 500 μm in K, L. M. Quantification of EdU labeled cells in large wounds compared to nearby unwounded areas in the same oral siphon. EdU labeled cells were counted in 400 μm2 areas. N: 8 wounded animals. Error bars represent SD. P < 0.000. Statistics was carried out by Student’s t test.
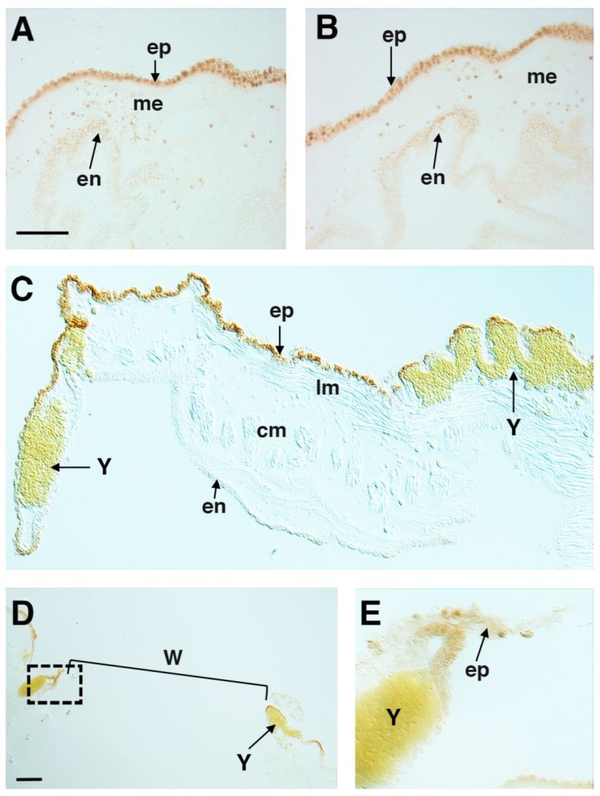
Figure 6.
Apoptosis during distal regeneration and wound repair. A, B. TUNEL labeled cells in the epidermal epithelium (ep) and to a lesser extend in the mesenchyme (me) at 1 (A) and 0.5 (B) days after amputation of the oral (A) and atrial (B) siphons. en: endodermal epithelium. Sections are cut perpendicular to the distal-proximal axis of each siphon. C. TUNEL labeled cells the epidermal epithelium (ep) of a wounded oral siphon 1 day after the operation. Yellow pigment cells (Y) congregate in the mesenchyme at the wound site. lm: longitudinal muscle bands. cm: circular muscle bands. Section is cut parallel to the distal-proximal axis of the wounded siphon. Scale bar: 10 μm in A-C. D-E. TUNEL labeled cells at 1 day after neural complex removal. D shows a low magnification image of a section parallel to the wound (W) showing the tissue gap (bracket), and a boxed area at one margin of the gap, which is magnified approximately 3.5X in E to show apoptotic cells in the epidermal epithelium (ep) at the margin of the wound. Y: Yellow pigment cells (Y) aggregate near the wounds. Scale bar: 10 μm in D.
Previous studies have shown that wounds can produce supernumerary siphons in Ciona (von Haffner, 1933). Likewise, it was found here that animals with open, but not sealed wounds, produced small or large supernumerary siphons rimmed with OPO (Fig. 4G, H). After 4 days of chase, higher levels of EdU labeling were detected in tissues associated with the small and large wound sites than in the surrounding tissues, regardless of whether they had sealed or remained open or initially showed OPO (Figs. 4G, inset; 4I-M). Lower levels of labeling were detected in other parts of the oral siphon (Fig. 4I-M) and in the atrial siphon. No labeling was detected in the NC of wounded animals (Fig. 4I, J). The results show that distal wounding causes the activation and targeting of progenitor cells to wound sites and is associated with the formation of extra siphons, although progenitor cell accumulation at the wound site does not predict the induction of a supernumerary siphon.
Specific targeting of branchial sac progenitor cells during neural complex regeneration
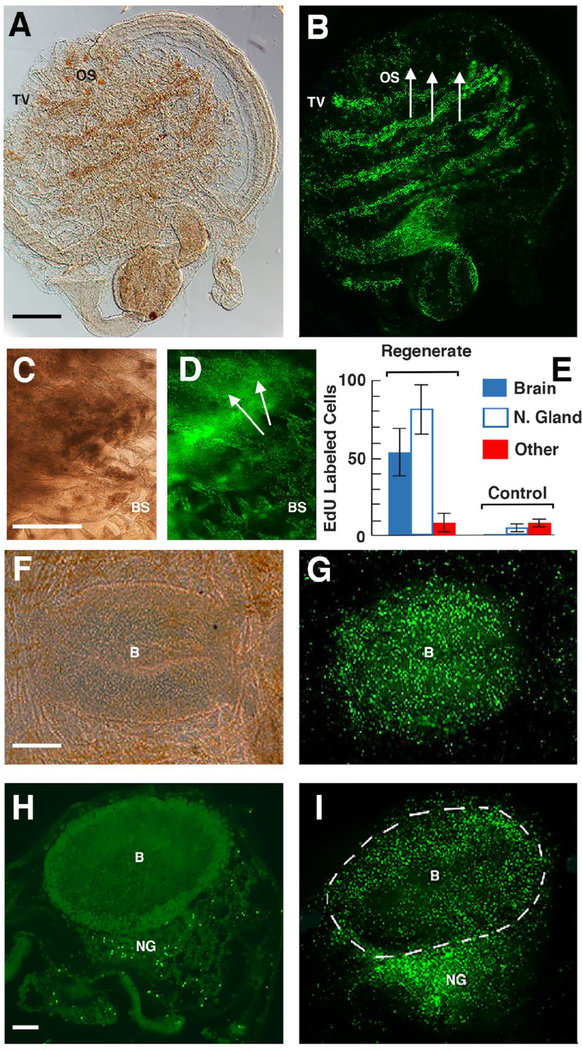
To address the possibility that progenitor cells from the branchial sac stem cell niche are involved in NC regeneration, the NC was ablated and the animals lacking NCs and sham operated controls were subjected to the standard EdU labeling period followed by 5, 20, or 40 days of chase. As observed during siphon amputation and injury (Figs. 2–4), NC removal resulted in high EdU labeling in the transverse vessels of the branchial sac, but not in any of the distal areas of the body, including the siphons and the region around the wounds produced by NC removal (Fig. 5A, B). By day 5 of the chase, an opaque region where the original NC was located, but not the surrounding tissue (except for the underlying branchial sac), showed EdU incorporation (Fig. 5C, D). This labeled region probably corresponds to the EdU labeled blastema previously observed during early NC regeneration (Dahlberg et al., 2009). At 25-days post-chase, when NC regeneration was in progress, the regenerating NC, but not the surrounding tissues, showed high levels of EdU labeling (Fig. 5E, G). Likewise, after NC regeneration was complete at 40 days of chase, EdU labeling was seen only in the NC, with high concentrations of EdU labeled cells detected in both the neural gland and brain. Quantification at this time showed that the density of EdU labeled cells was significantly higher in the neural gland than in the brain (p < 0.002), and both of the brain and neural gland showed significantly higher levels of EdU labeled cells than regions outside the NC (each p < 0.000) (Fig. 5E). Sham operated control animals also showed much lower levels of EdU labeling in the NC compared to regenerating animals (each p < 0.000) (Fig. 5E). The results suggest that progenitor cells from the branchial sac are activated after NC removal and specifically target the regenerating NC to populate the neural gland and brain with new cells.
Figure 5.
Branchial sac progenitor cells target the regenerating neural complex. A, B. Lateral views of the same animal after neural complex removal and the standard 24 hr EdU labeling period. OS: oral siphon. Arrows: area of neural complex removal. TV: EdU labeled transverse vessels. Scale bar: 100 μm in A, B. C, D. Dorsal view showing blastema of EdU labeled cells at the site of neural complex ablation (arrows) 5 days after the end of the chase. BS: branchial sac. Scale bar: 40 μm in C, D. E. Quantification of EdU labeled cells in sections of animals with regenerated NC and sham operated controls after 40 days of chase. EdU labeled cells were counted in 400 μm2 areas of the brain, neural gland, and an area outside the neural complex. N of regenerating animals: 8. F, G. N of sham operated controls: 6. Error bars represent SD. Statistics were done by Student’s t tests, resulting in the p values mentioned in the text. F, G. Dorsal view of EdU labeling in the regenerating neural complex following 20 days of chase. B: brain. Scale bar: 15 μm in F, G. H, I. Fluorescence images of EdU labeling in sections of a sham-operated control (H) and an animal with a regenerated neural complex (H) following 40 days of chase. Scale bar: 10 μM in H, I. B: Brain. NG: Neural gland. A, C, F. Bright field images. B, D, G-I. Fluorescence images.
Apoptosis during distal regeneration and wound repair
The stigmata targeted by progenitor cells from the branchial sac stem cell niche exhibit apoptosis during homeostasis (Fig. 1), suggesting that cell death and turnover could be involved in the attraction of progenitor cells to their targets. To address this possibility, we asked whether apoptosis occurs at other sites targeted by branchial sac progenitor cells during wound repair and distal regeneration. Animals with amputated oral or atrial siphons, wounds in the oral siphon, and extirpated NCs were fixed 0.5–1 day after the operations, subjected to TUNEL, and sectioned to detect apoptotic cells. As shown in Figure 6, apoptosis occurred after each type of operation. Zones of TUNEL positive cells were detected in the epidermal epithelia, and to a lesser extent in the mesenchyme, at the sites of oral or atrial siphon regeneration (Fig 6A, oral siphon wounds (Fig. 6C), and NC ablation (Fig. 6D). The results show that apoptosis begins before the targeting of progenitor cells to the sites of wound repair and distal regeneration.
Discussion
This study has explored the targeting and functional diversity of progenitor cells derived from an adult stem cell niche in the solitary ascidian Ciona intestinalis. The results reveal the multiple roles of progenitor cells derived from branchial sac stem cell niches in homeostasis, wound repair, and siphon and central nervous system regeneration, as well as a high degree of precision in selecting their appropriate targets (see Fig. 7 for summary).
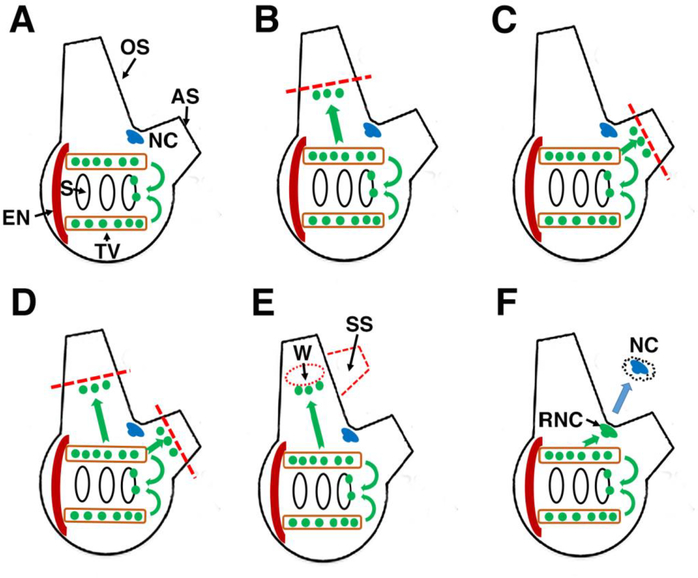
Figure 7.
Summary of progenitor cell targeting in Ciona inferred from EdU pulse-chase experiments. A. During homeostasis progenitor cells move from the stem cell niches of the branchial sac in the stigmata, which also occurs during distal regeneration and wound repair (BF). Green spheres: progenitor cells. Green arrows: Inferred migration of progenitor cells. OS: oral siphon. AS: atrial siphon. NC: neural complex. S: Stigmata. TV: Transverse vessel of the branchial sac. EN: endostyle. B, C. After oral or atrial siphon amputation, progenitor cells specifically target the regenerating oral or atrial siphons, respectively. D. After amputation of the oral and atrial siphons, progenitor cells target both siphons. E. After the oral siphon is wounded, progenitor cells target the wound site (W), and a supernumerary siphon (SS) may form. F. After the neural complex is removed, progenitor cells target the regenerating neural complex (RNC). Blue arrow: Neural complex ablation.
During homeostatic growth, progenitor cells target the stigmatal cells of the branchial sac (Fig. 7A). The stigmata are bordered by ciliated cells that beat continuously to drive water through the pharynx during feeding and respiration. These cells are likely to experience damage through “wear and tear” and have a correspondingly high rate of turnover and replacement, which is facilitated by stem cell niches in the surrounding transverse vessels of the branchial sac. In regenerating or wounded animals, these stem cell niches appear to function simultaneously in replacing the stigmatal cells and dispatching progenitor cells to sites of distal injury. This multitasking suggests a high capacity for progenitor cell production and is consistent with strong EdU labeling of the stem cells, even after brief EdU pulses (Jeffery, 2015b).
During regeneration of the oral and atrial siphons (Fig. 7B, C), progenitor cells are responsible for the development of the blastema, which primarily functions in the replacement of circular muscle fibers in the siphon mesenchymal layer. The circular muscle fibers and any associated satellite cells are completely removed when a siphon is amputated at its base (Auger et al., 2010). Therefore, progenitor cells from the regeneration blastema may play a key role in the rapid recovery of siphon contraction following injury. Coelomic cells with features resembling the progenitor cells involved in Ciona siphon regeneration also differentiate into muscle cells in colonial ascidians (Sugino et al., 2007). Underscoring their high capacity for replacement, the branchial sac stem cell niches supply progenitors to both the oral and atrial siphons after double amputations (Fig. 7D). However, during single siphon amputations, progenitor cells are targeted only to the injured siphons (Fig. 7B, C), highlighting the specificity of progenitor cell targeting.
Progenitor cells from the branchial sac also target the sites of wound repair in the oral siphon, which sometimes includes the induction of a supernumerary siphon (Fig. 7E). The development of extra siphons at wound sites is a curious phenomenon that has been encountered throughout the history of Ciona regeneration research (von Haffner, 1933; Jeffery, 2015c), and has also been reported in other ascidians (George, 1937). Although the present investigation showed that the invasion of progenitor cells from the branchial sac precedes the formation of supernumerary siphons, their presence at the wound site does not predict whether an extra siphon will be formed. Progenitor cells also target wounds that eventually seal and do not form supernumerary siphons. Therefore, the cue for supernumerary siphon induction is still an open question.
Complete brain regeneration appears to be a novel property of the ascidian (or tunicate) chordates. The vertebrates exhibit more restricted capacities for brain replacement, usually involving only small parts of the brain (Gage, 2002; Endo et al. 2007). NC regeneration has been studied for many years in Ciona, but the stem and progenitor cells involved in this process have remained elusive (Jeffery, 2015c). The results of the present investigation provide evidence for the involvement of progenitor cells from the branchial sac in NC regeneration (Fig. 7F). Considering the large number of EdU labeled cells seen in the regenerating brain and neural gland, it is likely that most or all the cells of these organs are derived from branchial sac stem cells. It is remarkable that the adult stem cell niches supplying the progenitor cells for NC regeneration are also used during homeostasis and regeneration/repair of other distal tissues and organs, although it remains to be demonstrated whether or not exactly the same branchial sac niches are mobilized in each of these processes. The results suggest that progenitor cells derived from the branchial sac are collectively multipotent and can differentiate into neural, muscle, and perhaps other cell types.
Non-chordate invertebrates often use multipotent blood cells to replace neurons in the central nervous system (da Silva et al., 2015). For example, the precursors for adult neurogenesis in the crayfish brain are supplied by a stem cell niche outside of the nervous system, which is populated by blood-born cells that are able to differentiate into neurons (Benton et al., 2014). Thus, in striking contrast to their vertebrate sister group, the ascidians may have conserved an ancient invertebrate style mechanism for brain neurogenesis and regeneration based on the utilization of blood-cell like progenitors. If so, the evolution of a blood-brain barrier may have been the critical vertebrate innovation invoking restrictions in brain regeneration.
The branchial sac is the largest organ by volume in the Ciona body (Fig. 1A; Millar, 1953) and contains numerous transverse blood vessels, each containing multiple nodules harboring stem cell niches. In contrast, the blastemas of proliferating cells formed at the locations of distal injury and regeneration are much smaller, implying that the major function of branchial sac stem cells may be in homeostatic growth. The demonstration that branchial sac stem cell niches are the source of new ciliated cells lining the stigmata, which may number in the hundreds in a mature Ciona, can explain the necessity for a large number of stem cell niches in the branchial sac. The branchial sac stem cells may also be responsible for the proliferation of hemocytes (Ermak, 1976; Cooper, 2009), a heterogeneous group of cell types that migrate through open tissue spaces and channels throughout the adult ascidian body and tunic (Rowley, 1981, Millar, 1953, Satoh, 1994). It will be important to identify other tissues and organs with high turnover rates in Ciona and to determine whether their source is the branchial sac or another adult stem cell niche.
The endostyle, a secretory organ embedded in the ventral margin of the branchial sac with homology to the mammalian thyroid gland (Ogasawara et al., 1999), contains a niche of adult stem cells in the colonial ascidian Botryllus (Voskoboynik et al., 2008). In the present and previous investigation (Jeffery, 2015b), the endostyle of Ciona did not show positive staining with PIWI and AP stem cell markers or label during a short EdU pulse, as expected of an organ actively producing progenitor cells. However, in a few cases the presence of EdU labeled cells was reported in the longitudinal blood vessels of the endostyle in the chase following oral siphon amputation (Jeffery, 2015b). This observation was confirmed here, and it was also noted that endostyle labeling is more frequent after both siphons are amputated and regenerating, possibly because the additional progenitor cells required for double siphon replacement are more easily detectable by our methods. The delayed labeling of the endostyle after an EdU pulse suggests that in Ciona progenitor cells may transit to their distal targets via the longitudinal blood sinuses in this organ, which are connected laterally to the transverse vessels of the branchial sac (Millar, 1953). An alternative solution for the possible differences in adult stem cell niche localization in Ciona and colonial ascidians is that their position may have changed during evolution. The Botryllid ascidians, which are derived from solitary ascidian ancestors (Zeng et al., 2006), may have consolidated adult stem cell niches in or near the endostyle to conserve space in their small and continuously recycling zooids.
The precision of progenitor cell targeting in homeostatic growth and injury requires a mechanism in which migratory cells are attracted to diverse areas of the body. In many different animals, apoptosis is considered to be the driving force for homoeostasis and regenerative activity (Bermann and Steller, 2010). Therefore, it was intriguing to discover that apoptosis occurs in all of the areas targeted by branchial sac progenitor cells: the stigmata during homeostasis, the sites of tissue repair during wound healing, and the blastemas formed during siphon and NC regeneration. A similar relationship exists between apoptosis/tissue disintegration and stem cell mobilization and migration in other animals; for example, during the replacement of dying tracheae during Drosophila metamorphosis (Chen and Krasnow, 2014). The following hypothesis is proposed to explain the precision of progenitor cell targeting from the branchial sac stem cell niche to locations where new cells are needed in Ciona. Tissues that are normally replaced at high rates or are injured may secrete diffusible signals triggered by apoptosis, which mobilize cell division in the branchial sac stem cell niches and guide the movement of progenitor cells to the sites of tissue replacement. There are some reasonable candidates for the molecular identity of these signals. In Botryllus, the lipid signaling molecule sphingosine-1-phosphate has been shown to attract the migration of germ line stem cells to new locations in the secondary buds (Kassmer et al., 2015). Another candidate is the netrin family of guidance molecules that serve as extracellular cues directing the migration of diverse cell types (Sun et al., 2011). The expression of a netrin gene is increased several fold as an early step in Ciona siphon regeneration (Hamada et al., 2015). Other candidates are factors that are upregulated during siphon regeneration, such as cell cycle regulators and growth factor ligands (Hamada et al., 2015; Spina et al., 2017).
In conclusion, Ciona is a relatively simple and tractable model for studying the mechanisms of progenitor cell migration and future studies with this animal can be expected to improve our understanding of the molecular cues involved in the replacement of tissues and organs by adult stem cells. The Ciona model system is now positioned to answer fundamental questions relate4d to the connections between apoptotic cell death, the activation of proliferation in adult stem cells, and the precision of progenitor cell migration during tissue turnover and replacement.
Highlights.
Branchial sac stem cells are mobilized to produce progenitor cells during homeostasis, wound healing, and regeneration of the siphons and neural complex
Migratory progenitor cells specifically target the sites of tissue replacement
Progenitor cells are multipotent
Progenitor cell targeting is correlated with apoptosis at the sites of tissue replacement
Acknowledgements
We thank Amy Parkhurst for technical assistance. This research was supported by National Institutes of Health grant AG055411.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auger H, Sasakura Y, Joly J-S, Jeffery WR. Regeneration of oral siphon pigment organs in the ascidian Ciona intestinalis. Dev Biol. 2010; 339:374–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Kery R, Li J, Noonin C, Soderhall I, Beltz BS. Cells from the immune system generate adult-born neurons in crayfish. Dev Cell 2014; 30:322–333. [DOI] [PubMed] [Google Scholar]
- Bergmann A, Steller H. Apoptosis, stem cells, and tissue regeneration. Sci Signal. 2010; 3: DOI: 10.1126/scisignal.3145re8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrill NJ. Development of Ciona. J Mar Biol Assoc. 1947; 26:616–625 [DOI] [PubMed] [Google Scholar]
- Berrill NJ. Regeneration and budding in tunicates. Biol Rev. 1951; 26:456–475. [Google Scholar]
- Bollner T Recent trends in regeneration research New York: Plenum Press; 1989. Regeneration of the central nervous system of the ascidian Ciona intestinalis. Pp. 281–287. [Google Scholar]
- Bollner T, Beesley PW, Thorndyke MC. Investigation of the contribution from peripheral GnRH-like immunoreactive ‘neuroblasts’ to the regenerating central nervous system in the protochordate Ciona intestinalis. Proc Roy Soc B. 1997; 264:1117–1123. [Google Scholar]
- Brien P Blastogenesis and morphogenesis. Adv Morphog. 1968; 7:151–203. [DOI] [PubMed] [Google Scholar]
- Brown FD, Keeling EL, Le AD, Swalla BJ. Whole body regeneration in a colonial ascidian, Botrylloides violaceus. J Exp Zool Mol Dev Evol. 2009; 312B:885–900. [DOI] [PubMed] [Google Scholar]
- Candia Carnevali DM, Burighel P. Encyclopedia of Life Sciences (ELS) John Wiley and Sons, Chichester, UK: 2010. Regeneration in echinoderms and ascidians. DOI: 10.1002/9780470015902 [DOI] [Google Scholar]
- Chaves da Silva PG, Santos de Abreu I, Cavalcante LA, Monteiro de Barros C, Allodi S. Role of hemocytes in invertebrate adult neurogenesis and brain repair. Invert Surv J. 2015; 12:142–154. [Google Scholar]
- Chen F, Krasnow MA. Progenitor outgrowth from the niche in Drosophila trachea is guided by FGF from decaying branches. Science 2014; 343:186–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Sasaki A, Nakayama A, Takamura K, Satoh N. Development of Ciona intestinalis juveniles (Through 2nd ascidian stage). Zool Sci. 2004; 21:285–298. [DOI] [PubMed] [Google Scholar]
- Cooper EL. Stem Cells in Marine Organisms Springer, Dordrecht, 2009; Putative stem cell origins in solitary tunicates. Pp 21–32. [Google Scholar]
- Dahlberg C, Auger H, Dupont S, Sasakura Y, Thorndyke M, Joly J-S. Refining the model system of central nervous system regeneration in Ciona intestinalis. PLoS One. 2009; 4(2):e4458. doi: 10.1371/journal.pone.0004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyts C, Casane D, Vernier P, Bourrat F, Joly J-S Morphological and gene expression similarities suggest that the ascidian neural gland may be osmoregulatory and homologous to vertebrate per-ventricular organs. Eur J Neurosci. 2006; 24:2299–2308. [DOI] [PubMed] [Google Scholar]
- Endo T, Yoshino J, Kado K, Tochinai S. 2007. Brain regeneration in amphibians. Develop Growth Diff 1007; 49:121–129. [DOI] [PubMed] [Google Scholar]
- Ermak TH Phylogeny of thymus and bone marrow–bursa cells Elsevier/North Holland, Amsterdam: 1976. The hematogenic tissues of tunicates. Pp. 45–56. [Google Scholar]
- Fedele M Il sistema nervoso degli `Ascidiacea’ nel piano di organizzazione dell’ Cordati Atti del’ Academ Naìionale Lincei Rendiconti Series. 1938; 6:370–376. [Google Scholar]
- Freeman G The role of blood cells in the process of asexual reproduction in the tunicate Perophora. J. Exp. Zool 1964; 156:157–184. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J. Neurosci 2002; 22:612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George WC. The formation of new siphon openings in the tunicate, Styela plicata. J Elisha Mitchell Sci Soc. 1937; 53:87–91. [Google Scholar]
- Haffner von K Die Uberzaligen Siphon und Ocellen von Ciona intestinalis L. (Experimentellmorpholosiche Untersunchungen). Z Wiss Zool. 1933; 143:16–52. [Google Scholar]
- Hamada M, Goricki S, Byerly MS, Satoh N, Jeffery WR. Evolution of the chordate regeneration blastema: Differential gene expression and conserved role of notch signaling during siphon regeneration in the ascidian Ciona. Dev Biol. 2015; 405:304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschler J Uber die Restitutions-und Involutions- vorange bei operierten Exemplaren von Ciona intestinalis Flem. (Teil I) nebst Bemurkungen uber den Wert des Negativen fur das Potenzproblem. 1914; Arch mikr Anat. 85:205–227. [Google Scholar]
- Jeffery WR. The tunicate Ciona: a model system for studying the relationship between regeneration and aging. Invert Reprod Dev. 2015a; 59:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery WR. Distal regeneration involves the age-dependent activity of branchial sac stem cells in the ascidian Ciona intestinalis. Regeneration 2015b; 2:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery WR. Closing the wounds: One hundred and twenty five years of regenerative biology in the ascidian Ciona intestinalis. Genesis 2015c; 53:48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery WR, Chiba T, Razy-Krajka F, Deyts C, Satoh N, Joly J-S. Trunk lateral cells are neural crest-like cells in the ascidian Ciona intestinalis: insights into the ancestry and evolution of the neural crest. Dev Biol. 2008; 324:152–160. [DOI] [PubMed] [Google Scholar]
- Kassmer SH, Rogriguez D, Langenbacher AD, Bul C, De Tomaso AW. Migration of germ line progenitor cells is directed by sphingosine-1-phosphate signaling in a basal chordate. Nat Commun. 2015; 6:8565 DOI: 10.1038/ncomms956510.1038/ncomms9565www.nature.com/naturecommunicationswww.nature.com/naturecommunications [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas de B, Perez LM, Galvez BG. Importance and regulation of adult stem cell migration. J Cell Mol Med. 2018; 22:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni L, Lane NJ, Zaniolo G, Burighel P. Cell reorganization during epithelial fission and perforation: The case of the ascidian branchial fissure. Dev Dyn. 2002; 234:303–313. [DOI] [PubMed] [Google Scholar]
- Medina BN, de Abreu IS, Cavalcante LA, Silva WAB, da Fonseca RN, Allodi S, de Barros CM. 3-acetylpyridine-induced degeneration in the adult ascidian neural complex: reactive and regenerative changes in glia and blood cells. Dev Neurobiol. 2014; DOI 10.1002/dneu.22255. [DOI] [PubMed] [Google Scholar]
- Millar RH. 1953. LMBC Memoirs on Typical British Marine Plants and Animals Liverpool University, Liverpool, U.K, Ciona: Pp. 1–123. [Google Scholar]
- Ogasawara M, Di Lauro R, Satoh N. Ascidian homologs of mammalian thyroid transcription factor-1 gene are expressed in the endostyle. Zool Sci. 1999; 16:559–565. [DOI] [PubMed] [Google Scholar]
- Osugi T, Sasakura Y, Satake H. The nervous system of the adult ascidian Ciona intestinalis Type A (Ciona robusta): Insights from transgenic animals. PLoS One 2017; 12(6): e0180227 10.1371/journal.pone.0180227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Rosner A, Rabinowitz C, Lapidot Z, Moiseeva E, Rinkevich B. Piwi positive cells that line the vasculature epithelium underlie whole body regeneration in a basal chordate. Dev Biol. 2010; 345:94–104. [DOI] [PubMed] [Google Scholar]
- Rowley AF. The blood cells of the sea squirt, Ciona intestinalis: morphology, differential counts, and in vitro phagocytotic activity. J Invert Pathol.1981; 37:91–100. [Google Scholar]
- Satoh N Developmental Biology of Ascidians. Cambridge University Press, Cambridge: 1994. [Google Scholar]
- Schultze LS. Die Regeneration des Ganglions von Ciona intestinalis L. Jena Z f Naturwiss. 1899; 33:263–344. [Google Scholar]
- Shimazaki A, Sakai A, Ogasawara M. Gene expression profiles in Ciona intestinalis stigmatal cells: Insight into formation of the ascidian branchial fissures. Dev Dyn. 2006; 235:562–569. [DOI] [PubMed] [Google Scholar]
- Sugino Y, Matsumura M, Kawamura K. Body muscle-cell differentiation from coelomic stem cells in colonial tunicates. Zool. Sci 2007; 24:542–546. [DOI] [PubMed] [Google Scholar]
- Sun KLW, Correla JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development 2011; 138:2153–2169. [DOI] [PubMed] [Google Scholar]
- Spina EJ, Guzman E, Zhou H, Kosik KS, Smith WC. A microRNA-mRNA expression network during oral siphon regeneration in Ciona. Development 2017; 144: 1787–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiozzo S, Brown FD, De Tomaso AW. Stem Cells: From Hydra to Man, 2008. Springer, New York, Chapter 6. Regeneration and stem cells in ascidians. Pp. 95–112. [Google Scholar]
- Zeng L, Jacobs MW, Swalla BJ. Coloniality has evolved once in Stolidobranch ascidians. Integr Comp Biol. 2006; 46:255–268. [DOI] [PubMed] [Google Scholar]
- Voskoboynik A, Soen Y, Rinkevich Y, Rosner A, Ueno H, Reshef R, Ishizuka KJ, Palmeri KJ, Moiseeva E, Rinkevich B, Weissman IL. Identification of the endostyle as a stem cell niche in a colonial chordate. Cell Stem Cell 2008; 3:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker JR. Siphon regeneration in Ciona. 1975; Nature 255:224–225. [DOI] [PubMed] [Google Scholar]