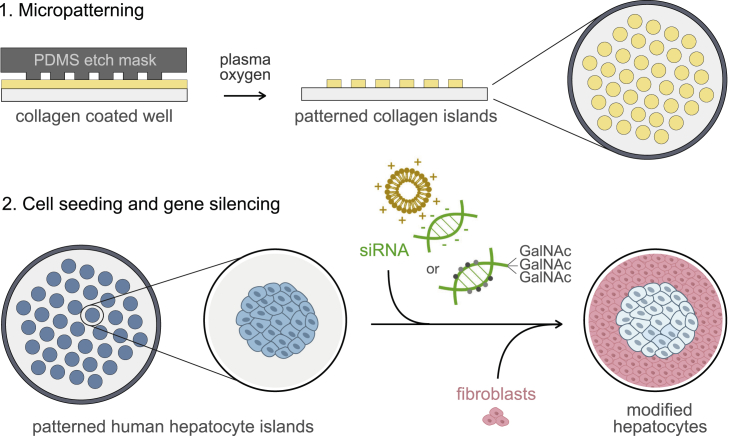
Figure 1.
Fabrication of Gene-Silenced MPCCs
Fabrication of MPCCs involves a two-step process: first, patterning of collagen islands in multi-well plates using photolithography techniques, and second, seeding of primary human hepatocytes and stromal supportive fibroblasts to establish the co-culture system. Patterned collagen islands (spots of 500 μm diameter; illustrations represent side and top views of the well) are obtained by applying a silicone elastomer polydimethylsiloxane (PDMS) mask to collagen (yellow)-coated wells, followed by exposure to oxygen plasma. Next, primary human hepatocytes (blue) are seeded and allowed to settle onto the collagen, creating patterned hepatocyte islands (top view). Nucleic acids (siRNA, green) are then included in the media and incubated for 20–24 h, after which mouse fibroblasts (pink) are seeded and allowed to bind in the intervening space between hepatocyte islands. Regular negatively charged siRNAs can be delivered to hepatocytes using cationic lipid transfection reagents, such as lipofectamine (orange). Chemically stabilized siRNAs conjugated to GalNAc are delivered to hepatocytes via interaction with the hepatocyte surface receptor, ASGPR. RNAi-induced gene silencing (light blue) efficiency can be assessed by multiple functional readouts (ELISA for secreted proteins, microscopy or flow cytometry for protein expression, RT-PCR for mRNA transcript abundance, or enzymatic cell assays) and challenged by different experimental perturbations, such as drugs or hepatotropic pathogens (e.g., Plasmodium parasites and hepatitis B or C viruses).