Abstract
Background
Soil-transmitted helminthes (STH) infections are among the most common infections worldwide and affect the most deprived communities. Adequate water, sanitation, and hygiene (WASH) prevents environmental contamination, thereby preventing transmission of STH. Cognizant of this, WASH education was implemented in rural Dembiya to reduce intestinal parasitic infections. This study was, therefore, conducted to assess the impacts of the intervention on households’ WASH conditions and prevalence of intestinal parasitic infections.
Method
An uncontrolled before-and-after intervention study was used. Cross-sectional studies were done before and after the intervention. Two hundred twenty-five and 302 under five children were recruited randomly at the baseline and endline, respectively. Data were collected using a structured questionnaire and observational checklists. Direct stool examination and Kato-Katz methods were used to identify parasites in the stool. We used percent point change and prevalence ratio (PR) to see the effects of the intervention on WASH conditions and prevalence of intestinal parasitic infections respectively. Pearson chi-squared and Fisher’s exact tests were used to test for statistically significant percentage point changes of WASH conditions. The effect of the intervention on intestinal parasitic infections was statistically tested on the basis of PR with 95% confidence interval (CI).
Results
The baseline prevalence of intestinal parasitic infections was 25.8%, and the endline prevalence was 23.8%. The prevalence of intestinal parasitic infections was not significantly decreased at the endline compared with the baseline [PR = 0.92, 95% CI = (0.62, 1.38)]. Ascaris Lumbricoides was the most prevalent parasitic infection both at the baseline and endline. The proportion of children who had good hygienic condition increased from 1.3% at the baseline to 34.4% at the end line (p < 0.05). The percentage of mothers/care givers who washed hands at different pick times was significantly increased from 24.4% at the baseline to 68.2% at the endline (p < 0.001). The proportion of households who practiced home-based water treatment was significantly increased from 7.6% at the baseline to 47% at the endline (p < 0.001). The proportion of households who used sanitary latrine was increased from 32% at the baseline to 49% at the endline (p < 0.05).
Conclusion
This before-and-after intervention study found that households’ WASH performance was significantly improved at the endline compared with the baseline. The endline prevalence of intestinal parasitic infections was slightly lower than the baseline prevalence; however, the reduction was not statistically significant. The local health office needs to strengthen the WASH education program, mobilize the community to construct WASH facilities, and support the community to sustain households’ WASH performance.
Keywords: Intestinal parasitic infections, WASH education, Children aged 6–59 months, Uncontrolled before and after intervention study, Rural Dembiya
Background
Soil-transmitted helminthes (STH) infections are among the most common infections worldwide and affect the poorest and most deprived communities. They are transmitted via eggs present in human feces, which contaminate soil in areas where sanitation is poor. The most common STH infections are roundworm (Ascaris lumbricoides), whipworm (Trichuris trichiura), and human hookworm (Necator americanus and Ancylostoma duodenale) [1–5].
The global health impact of STH infections was ranging between 4 million and 39 million disability adjusted life years (DALYs) [6]. In 2010, at least 1.3 billion people were estimated to be infected with at least one STH species [7]. The majority of the disease burden associated to STH infections is understood to be in children [8, 9] where infections are acquired through playing with contaminated soil and pica habits [10, 11]. In 2010, World Health Organization (WHO) estimated that 875 million children needed regular STH treatment [12]. STH infections in children can lead to under nutrition and growth faltering [7, 13–15], impair cognitive development [16–19], and cause anemia [20–23].
Intestinal parasitic worms enter the human host either through penetration of the skin or ingestion from contaminated hands or agricultural products [24–26]. Adequate sanitation prevents release of feces into the environment, thereby preventing transmission. Any single or combined water, sanitation, and hygiene (WASH) intervention reduces the risk of STH infections. A systematic review for the effect of latrine availability and utilization on STH infections found that latrine utilization reduced the risk of combined STH infection by about 50% [27]. A more recent review considered all WASH interventions found that WASH access and practices were generally associated with reduced odds of STH infection [28]. Studies provided strong evidence linking hygiene practices especially hand washing with reductions in STH infection [29–32]. WASH education is also one of the effective interventions to prevent and control transmission of intestinal parasitic infections [30, 31, 33, 34]. Cognizant of this, WASH education was implemented in rural Dembiya to reduce the prevalence of intestinal parasitic infections. The project was so called Dembiya NTD-WASH project. This study was, therefore, conducted to assess the impacts of the intervention on WASH and intestinal worms.
Method
An uncontrolled before and after intervention study was used to determine the effect of WASH education on prevalence of childhood intestinal parasitic infections. Cross-sectional studies were done before and after the intervention. The study setting, study population, sample size determination and sampling procedures, data collection procedures, measurement of study variables, and stool examination methods were presented in a baseline study which is published elsewhere [35]. The baseline data were collected in May 2017, and the endline data were collected in May 2018. The sample size was re-calculated for the endline survey considering the prevalence of parasitic infections reported in the baseline [35].
Description of the intervention
WASH education was given to the communities of five rural kebels (the lowest administrative unit in Ethiopia) and five rural schools. From five to six subgroups were formed in every kebele and school to make the health education interactive and manageable. We used school teachers, school children, university students, religious leaders, and health extension workers as WASH educators. The education was given every week for three months immediately after the baseline survey and every week for three months before the endline survey. Personal hygiene, effective hand washing practice, drinking water quality measures, food safety measures, waste management, health consequences of poor WASH, mode of transmission of intestinal parasitic infections, and prevention and control measures of intestinal parasitic infections were the content of the education. Role-play or drama, demonstration, group discussion, song, card-game, question and answer, and lecturing were the methodology we used. A total of 906 school children (399 females and 507 males) and 670 household heads participated in the five schools and kebeles in every week sessions.
In addition to health education, hand washing facilities from locally available materials were constructed in all schools. In every school, we put two to four jars with 25 l capacity and one to two plastic barrels with a capacity of 400 l to promote hand washing after visiting toilet. Hence, water is not available in all schools; students filled the containers every morning by shift. Environmental health clubs and school principals facilitated this task. We also prepared leaflets and posters to promote WASH. Leaflets were dispatched to school children and communities. The leaflets were more of pictorial to entertain illiterate individuals. What makes the living environment unhygienic, health consequences of unhygienic environment, and strategies to make the environment hygienic were the contents of the posters and leaflets.
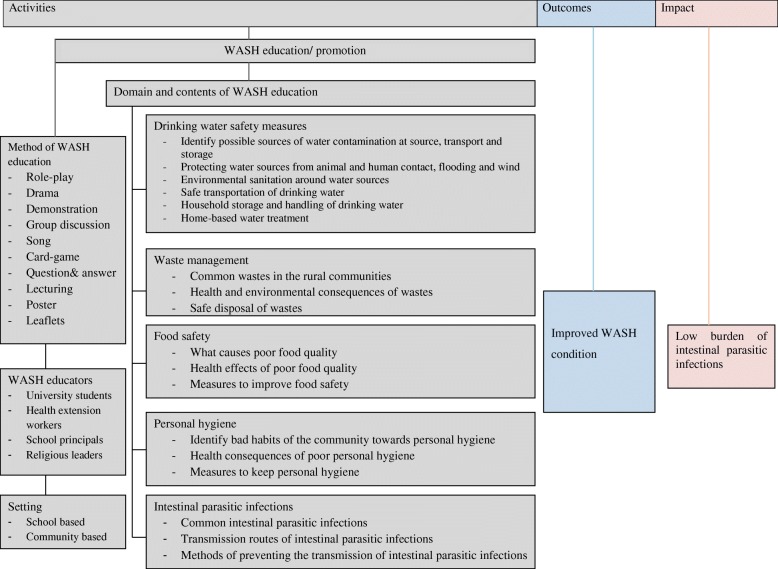
Weekly hygiene and sanitation supervision program was the other intervention we practiced. Home room teaches audit the hygiene condition of their students once a week and gave feedback to both the students and their families. Science teachers and environmental health clubs supervised the sanitation condition of the school environment every week and conducted sanitary campaign when necessary. We also established WASH committee in every kebeles. The committee comprised health extension worker, school principal, religious leaders, and kebeles administrator. The committee provided WASH education to the rural communities and supervised the hygiene and sanitation performances of the communities. Moreover, the committee mobilized the community and transferred health messages in public gatherings like community meeting and in churches (Fig. 1).
Fig. 1.
A frame work shows short summary of WASH intervention program in rural Dembiya, northwest Ethiopia
Indicators tracked
Drinking water safety measures, environmental sanitation, personal hygiene, and parasitic infections were the four domains we used to track the change due to the interventions. Table 1 shows the household-level indicators tracked by the study. The detail of the indicators is well explained elsewhere [35].
Table 1.
Household-level indicators by domain used to track changes due to the interventions in rural Dembiya, northwest Ethiopia
| Domains | Indicators |
|---|---|
| Drinking water safety | % of households with children under five that practiced one or more home-based water treatment methods |
| Sanitation | % of households with children under five using hygienic latrine facilities |
| Hygiene | % of children whose personal hygiene condition is clean |
| % of mothers or care givers whose hand washing practice is good | |
| Parasitic infections | % of under five children who had one or more intestinal parasitic infections |
Statistical analysis
Data were entered using EPI-INFO version 3.5.3 statistical package. Data were imported, merged (baseline and endline data sets), and analyzed in SPSS statistical software package version 20. Composite variables were constructed by summing or scoring data collected in both rounds. The detail of scoring is published elsewhere [35]. For most variables, data were presented by frequency and percentage. The level of WASH conditions and prevalence of intestinal parasitic infections at the baseline and endline were compared. Percent point change with corresponding p value and PR with 95% CI were used to see the effects of the intervention on WASH conditions and intestinal parasitic infections respectively. Pearson chi-squared test was used to test for statistically significant changes between WASH indicators at baseline and endline. Fisher’s exact test was used for tables with expected values less than 5. Indicators with p values less than 0.05 and a 95% CI of PR not containing 1 showed a statistically significant change between baseline and endline.
Role of the funding source
The funding organization of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Socio-demographic characteristics of study participants
Two hundred twenty-five and 302 children aged 6–59 months participated at the baseline and endline surveys, respectively. The majority of study participants were aged above 24 months at both surveys. The highest proportions (80% at the base line and 82.1% at the endline) of caregivers were illiterate (Table 2).
Table 2.
Socio-demographic characteristics of study participants in the baseline (May 2017) and endline (May 2018) surveys in rural Dembiya, northwest Ethiopia
| Socio-demographic variables | Baseline survey | Endline survey |
|---|---|---|
| n (%) | n (%) | |
| Sex of children | ||
| Male | 106 (47.1) | 158 (52.3) |
| Female | 119 (52.9) | 144 (47.7) |
| Age of children | ||
| 6–24 | 59 (26.2) | 96 (31.8) |
| > 24 | 166 (73.8) | 206 (68.2) |
| Maternal education | ||
| No formal education | 180 (80.0) | 248 (82.1) |
| Have formal education | 45 (20.0) | 54 (17.9) |
WASH conditions
At baseline, the general hygienic condition of 1.3% of the children was good. Conversely, the percentage of children that had good hygienic condition increased to 34.4% at the endline (p < 0.05). The percentage of mothers/care givers who washed hands after visiting toilet or changing baby’s diaper or touching wastes, before eating and food preparation, was significantly increased from 24.4% at the baseline to 68.2% at the endline (p < 0.001). The proportion of households who used sanitary latrine was significantly increased from 32% at the baseline to 49% at the endline (p < 0.05). The proportion of households who practiced home-based water treatment was significantly increased from 7.6% at the baseline to 47% at the endline (p < 0.001) (Table 3). Water guard was the commonest home-based water treatment method practiced by the rural communities both at the baseline and endline (Table 4).
Table 3.
WASH condition before (May 2017) and after the intervention (May 2018) in rural Dembiya, northwest Ethiopia
| WASH variables | Baseline | Endline | % point change | p value |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Percentage of children whose personal hygiene condition is clean | 3 (1.3) | 104 (34.4) | 33.1 | < 0.05* |
| Percentage of mothers or caregivers whose hand washing practice is good | 55 (24.4) | 206 (68.2) | 43.8 | < 0.001 |
| Percentage of households practiced home-based water treatment | 17 (7.6) | 142 (47.0) | 39.4 | < 0.001 |
| Percentage of households used sanitary latrine | 72 (32.0) | 148 (49.0) | 17.0 | < 0.05 |
*Fisher’s exact test
Table 4.
Common home-based water treatment methods practiced before (May 2017) and after the intervention (May 2018) by the community of rural Dembiya
| Home-based water treatment methods | Baseline (n = 225) | Endline (n = 302) |
|---|---|---|
| n (%) | n (%) | |
| Water guard (chlorine solution) | 13 (5.8) | 142 (47.0) |
| Solar disinfection | 1 (0.4) | – |
| Boiling | 2 (0.9) | – |
| Cloth sieve filtration | 1 (0.4) | – |
| No treatment methods used | 208 (92.4) | 160 (53.0) |
Prevalence of intestinal parasitic infections
From a total of 225 children included in the baseline survey, 58 of the children were infected with one or more intestinal parasitic infections. The prevalence of intestinal parasitic infections at the baseline was, therefore, found to be 25.8%. One hundred children were unable to give stool sample at the endline. As a result, stool sample was taken from 202 children at the endline. Forty-eight children out of 202 were infected with one or more intestinal parasitic infections at the endline. The endline prevalence was, therefore, found to be 23.8%. At the endline, four children had double and two had triple infections. Ascaris Lumbricoides was the most prevalent parasitic infection both at the baseline and endline (Fig. 2). The prevalence of intestinal parasitic infections was not significantly decreased at the endline compared with the baseline [PR = 0.92, 95% CI = (0.62, 1.38)] (Table 5).
Fig. 2.
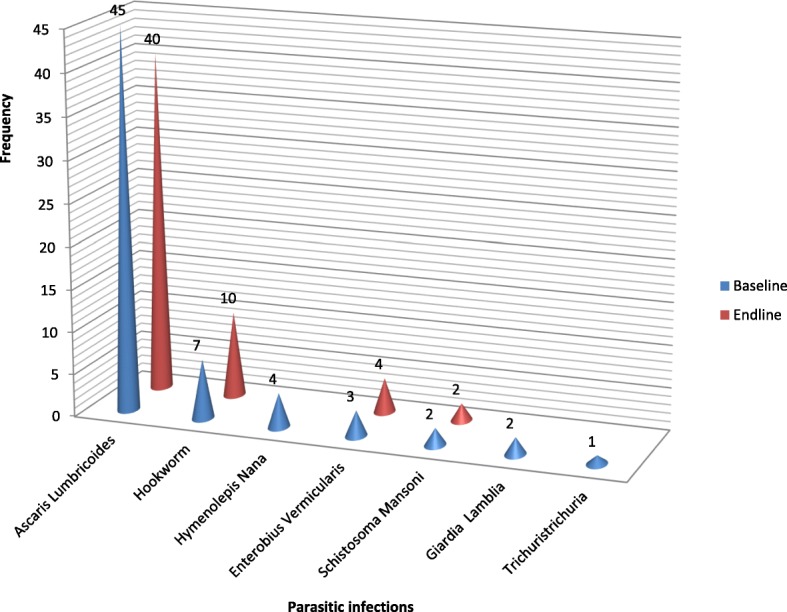
Common intestinal parasitic infections identified among children aged 6–59 months at the baseline (May 2017) and endline (May 2018) in rural Dembiya, northwest Ethiopia
Table 5.
The effect of WASH education on childhood intestinal parasitic infections in rural Dembiya, northwest Ethiopia
| Intestinal parasitic infections | Baseline (n = 225) | Endline (n = 202) | PR (95% CI) |
|---|---|---|---|
| n (%) | n (%) | ||
| Percentage of under five children who had at least one intestinal parasitic infection | 58 (25.8) | 48 (23.8) | 0.92 (0.62, 1.38) |
Discussion
The present study assessed the effects of WASH education on childhood parasitic infections in rural Dembiya, northwest Ethiopia. The prevalence of childhood intestinal parasitic infection was found 25.8% at the baseline and 23.8% at the endline. Children in the study area had developed one or more intestinal parasitic infections due to the fact that the population in the area had poor access to sanitation. During June 2017, clean water and latrine coverage in the district was 26.6% and 55% respectively [36]. The findings of the current study are similar with the reports of studies in Wonji Shoa Sugar Estate (24.3%) [37] and in Butajira town (23.3%) [38]. The baseline and endline prevalence of intestinal parasitic infections reported by this study is also lower than the findings of studies in Wondo Genet (85.1%) [39] and Hawassa Zuria District (51.3%) [40]. The lower prevalence might be due to the fact that anthelmintic drugs were administered by nongovernmental organizations in the current study area before the commencement of this project.
This study revealed that WASH education was significantly associated with households’ sanitation performance. Water safety measures, children’s hygiene condition, mothers’/care givers’ hand washing practice, and latrine utilization were significantly improved at the endline compared with the baseline conditions. The effect of WASH education on households’ WASH performance might be due to the fact that health education increases awareness on good WASH practices and encourages behavioral change [41–44]. The finding of this study is in line with intervention-based studies in Mali and India [45, 46]. However, the interventions in Mali and India were more effective than our intervention to promote households’ WASH performance due to the fact that the interventions in Mali and India were community-based total sanitation (CBTS) or community leading total sanitation (CLTS). CBTS or CLTS is one of the effective WASH promotion approaches to empower rural communities and to develop community ownership for better behavioral change [47–50].
Although it is not statistically significant, this intervention-based study reported that the prevalence of intestinal parasitic infections was slightly reduced at the endline compared with the baseline. This slight reduction might be due to the fact that WASH interventions have no curative effect once children are infected to the parasites. However, other similar studies reported significant reduction of intestinal parasitic infection due to WASH interventions [34, 45, 46]. This might be due to the fact that health education increases awareness about the potential health impacts of poor environmental sanitation and motivates the communities to take care for their health so that households implement barriers to prevent occurrence of infections [30, 31, 33, 51].
Lastly as a limitation, this research was uncontrolled before and after intervention study with no control group. The evidence generated may not be strong because of the weak nature of the research design. We could not clearly know whether our intervention or other activities brought WASH improvement and in the study area because of the absence of control group. We recommend randomized controlled trial studies in the area to generate concrete evidence on the link between WASH interventions and intestinal parasitic infections in the rural setups.
Conclusion
This before and after intervention study found that households’ WASH performance was significantly improved at the endline compared with the baseline. The endline prevalence of intestinal parasitic infections was slightly lower than the baseline prevalence; however, the reduction was not statistically significant. The local health office needs to strengthen the WASH education program, mobilize the community to construct WASH facilities, and support the community to sustain households’ WASH performance.
Acknowledgements
The authors are pleased to acknowledge data collectors, field supervisors and study participants for their unreserved contributions to the success of this study. The authors are also pleased to acknowledge NALA foundation for funding the project.
Funding
This Dembiya NTD-WASH project was funded by NALA foundation. NALA foundation is an Israel based nongovernmental organization developed into a thriving organization, working in many communities both on the ground and giving technical assistance to local authorities. NALA works together with local, national and international partners.
Availability of data and materials
Data will be made available upon requesting the primary author.
Abbreviations
- CBTS
Community-based total sanitation
- CI
Confidence interval
- CLTS
Community leading total sanitation
- DALYs
Disability adjusted life years
- NALA
Neglected tropical diseases advocacy learning action
- NTD
Neglected tropical diseases
- PR
Prevalence ratio
- SPSS
Statistical package for social sciences
- STH
Soil-transmitted helminthes
- WASH
Water, sanitation, and hygiene
- WHO
World Health Organization
Authors’ contributions
All the authors actively participated during conception of the research issue, development of a research proposal, data collection, analysis and interpretation, and writing various parts of the research report. ZG had designed the protocol, analyzed the data, supervised the overall research process, and prepared the manuscript. AA and HD had developed the data collection tools and supervised the data collection process. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethical clearance was obtained from the Institutional Review Board of the University of Gondar (ethics approval number is O/V/P/RCS/05/541/2017) and an official letter was submitted to the district administrators. There were no risks due to participation in this research project, and the collected data were used only for this research purpose. Verbal informed consent was obtained from the mothers/care givers. Information was kept with complete confidentiality. Appropriate anthelmintic drugs were given for children infected by intestinal parasitic infections together with brief health messages for the mothers or caregivers.
Consent for publication
This manuscript does not contain any individual person’s data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zemichael Gizaw, Email: zemichael12@gmail.com.
Ayenew Addisu, Email: ayenew.addisu@gmail.com.
Henok Dagne, Email: enoch2313@gmail.com.
References
- 1.Mascarini-Serra L. Prevention of soil-transmitted helminth infection. J Global Infect Dis. 2011;3(2):175. doi: 10.4103/0974-777X.81696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009;373(9674):1570–1575. doi: 10.1016/S0140-6736(09)60233-6. [DOI] [PubMed] [Google Scholar]
- 3.De Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19(12):547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367(9521):1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 5.Mitra A, Mawson A. Neglected tropical diseases: epidemiology and global burden. Trop Med Infect Dis. 2017;2(3):36. doi: 10.3390/tropicalmed2030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooker S. Estimating the global distribution and disease burden of intestinal nematode infections: adding up the numbers–a review. Int J Parasitol. 2010;40(10):1137–1144. doi: 10.1016/j.ijpara.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goto R, Mascie-Taylor CN, Lunn PG. Impact of intestinal permeability, inflammation status and parasitic infections on infant growth faltering in rural Bangladesh. Br J Nutr. 2009;101(10):1509–1516. doi: 10.1017/S0007114508083554. [DOI] [PubMed] [Google Scholar]
- 8.Brooker S, Clements AC, Bundy DA. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006;62:221–261. doi: 10.1016/S0065-308X(05)62007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galvani AP. Age-dependent epidemiological patterns and strain diversity in helminth parasites. J Parasitol. 2005;91(1):24–30. doi: 10.1645/GE-191R1. [DOI] [PubMed] [Google Scholar]
- 10.Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003;16(2):265–272. doi: 10.1128/CMR.16.2.265-272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher M. Toxocara cati: an underestimated zoonotic agent. Trends Parasitol. 2003;19(4):167–170. doi: 10.1016/s1471-4922(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 12.Jourdan PM, Lamberton PH, Fenwick A, Addiss DG. Soil-transmitted helminth infections. Lancet. 2018;391(10117):252–265. doi: 10.1016/S0140-6736(17)31930-X. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez AL, Gabrie JA, Usuanlele M-T, Rueda MM, Canales M, Gyorkos TW. Soil-transmitted helminth infections and nutritional status in school-age children from rural communities in Honduras. PLoS Negl Trop Dis. 2013;7(8):e2378. doi: 10.1371/journal.pntd.0002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crompton DWT, Nesheim M. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22(1):35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 15.Adams EJ, Stephenson LS, Latham MC, Kinoti SN. Physical activity and growth of Kenyan school children with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved after treatment with albendazole. J Nutr. 1994;124(8):1199–1206. doi: 10.1093/jn/124.8.1199. [DOI] [PubMed] [Google Scholar]
- 16.Nga TT, Winichagoon P, Dijkhuizen MA, Khan NC, Wasantwisut E, Wieringa FT. Decreased parasite load and improved cognitive outcomes caused by deworming and consumption of multi-micronutrient fortified biscuits in rural Vietnamese schoolchildren. Am J Trop Med Hyg. 2011;85(2):333–340. doi: 10.4269/ajtmh.2011.10-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ijaz MK, Rubino JR. Impact of infectious diseases on cognitive development in childhood and beyond: potential mitigational role of hygiene. Open Infect Dis J. 2012;6(1):65–70. [Google Scholar]
- 18.Ezeamama AE, Bustinduy AL, Nkwata AK, Martinez L, Pabalan N, Boivin MJ, et al. Cognitive deficits and educational loss in children with schistosome infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(1):e0005524. doi: 10.1371/journal.pntd.0005524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guernier V, Brennan B, Yakob L, Milinovich G, Clements AC, Magalhaes RJS. Gut microbiota disturbance during helminth infection: can it affect cognition and behaviour of children? BMC Infect Dis. 2017;17(1):58. doi: 10.1186/s12879-016-2146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koukounari A, Estambale BB, Njagi JK, Cundill B, Ajanga A, Crudder C, et al. Relationships between anaemia and parasitic infections in Kenyan schoolchildren: a Bayesian hierarchical modelling approach. Int J Parasitol. 2008;38(14):1663–1671. doi: 10.1016/j.ijpara.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oguntibeju OO. Parasitic infestation and anaemia: the prevalence in a rural hospital setting. J Indian Acad Clin Med. 2003;4:210–212. [Google Scholar]
- 22.Calvao F, Costa Gileno D, Malta JO, Vientini V, Anibal F. Anemia in patients with intestinal parasitic infection. Rev Ibero-Latinoam Parasitol. 2011;70(2):206–211. [Google Scholar]
- 23.Tsuyuoka R, Bailey JW, Guimarães AM, Gurgel RQ, Cuevas LE. Anemia and intestinal parasitic infections in primary school students in Aracaju, Sergipe, Brazil. Cadernos de Saúde pública. 1999;15(2):413–421. doi: 10.1590/s0102-311x1999000200026. [DOI] [PubMed] [Google Scholar]
- 24.Cox FE. History of human parasitology. Clin Microbiol Rev. 2002;15(4):595–612. doi: 10.1128/CMR.15.4.595-612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerba CP. Environmentally Transmitted Pathogens. In Environmental Microbiology: Third Edition. 2014 (pp.509-550). Elsevier Inc.. Available at 10.1016/B978-0-12-394626-3.00022-3.
- 26.Norhayati M, Fatmah M, Yusof S, Edariah A. Intestinal parasitic infections in man: a review. Med J Malays. 2003;58(2):296–305. [PubMed] [Google Scholar]
- 27.Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9(1):e1001162. doi: 10.1371/journal.pmed.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 2014;11(3):e1001620. doi: 10.1371/journal.pmed.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balen J, Raso G, Li Y-S, Zhao Z-Y, Yuan L-P, Williams GM, et al. Risk factors for helminth infections in a rural and a peri-urban setting of the Dongting Lake area, People’s Republic of China. Int J Parasitol. 2011;41(11):1165–1173. doi: 10.1016/j.ijpara.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Asaolu S, Ofoezie I. The role of health education and sanitation in the control of helminth infections. Acta Trop. 2003;86(2–3):283–294. doi: 10.1016/s0001-706x(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 31.Hosain GM, Saha S, Begum A. Impact of sanitation and health education on intestinal parasite infection among primary school aged children of Sherpur, Bangladesh. Trop Dr. 2003;33(3):139–143. doi: 10.1177/004947550303300306. [DOI] [PubMed] [Google Scholar]
- 32.Corrales LF, Izurieta R, Moe CL. Association between intestinal parasitic infections and type of sanitation system in rural El Salvador. Tropical Med Int Health. 2006;11(12):1821–1831. doi: 10.1111/j.1365-3156.2006.01737.x. [DOI] [PubMed] [Google Scholar]
- 33.Bieri FA, Gray DJ, Williams GM, Raso G, Li Y-S, Yuan L, et al. Health-education package to prevent worm infections in Chinese schoolchildren. N Engl J Med. 2013;368(17):1603–1612. doi: 10.1056/NEJMoa1204885. [DOI] [PubMed] [Google Scholar]
- 34.Gyorkos TW, Maheu-Giroux M, Blouin B, Casapia M. Impact of health education on soil-transmitted helminth infections in schoolchildren of the Peruvian Amazon: a cluster-randomized controlled trial. PLoS Negl Trop Dis. 2013;7(9):e2397. doi: 10.1371/journal.pntd.0002397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gizaw Z, Adane T, Azanaw J, Addisu A, Haile D. Childhood intestinal parasitic infection and sanitation predictors in rural Dembiya, northwest Ethiopia. Environ Health Prev Med. 2018;23(1):26. doi: 10.1186/s12199-018-0714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsigereda K, et al. Dembiya district health office annual report 2017. Koladiba: District health office, Amhara region, Ethiopia.
- 37.Degarege A, Erko B. Prevalence of intestinal parasitic infections among children under five years of age with emphasis on Schistosoma mansoni in Wonji Shoa Sugar Estate, Ethiopia. PLoS One. 2014;9(10):e109793. doi: 10.1371/journal.pone.0109793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shumbej T, Belay T, Mekonnen Z, Tefera T, Zemene E. Soil-transmitted helminths and associated factors among pre-school children in Butajira town, South-Central Ethiopia: a community-based cross-sectional study. PLoS One. 2015;10(8):e0136342. doi: 10.1371/journal.pone.0136342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyantekyi LA, Legesse M, Belay M, Tadesse K, Manaye K, Macias C, et al. Intestinal parasitic infections among under-five children and maternal awareness about the infections in Shesha Kekele, Wondo Genet, Southern Ethiopia. Ethiopian Journal of Health Development. 2010;24(3):185-190.
- 40.Kabeta A, Assefa S, Hailu D, Berhanu G. Intestinal parasitic infections and nutritional status of pre-school children in Hawassa Zuria District, South Ethiopia. Afr J Microbiol Res. 2017;11(31):1243–1251. [Google Scholar]
- 41.Bajracharya D. Myanmar experiences in sanitation and hygiene promotion: lessons learned and future directions. Int J Environ Health Res. 2003;13(sup1):S141–SS52. doi: 10.1080/0960312031000102903. [DOI] [PubMed] [Google Scholar]
- 42.Sriram A, Maheswari U. Integrated communication strategy for creating awareness on sanitation and hygiene behavior change. Int J Commun Health. 2013;14:1. [Google Scholar]
- 43.Naidoo N, Chidley C, McNamara A. The implementation of hygiene education programmes in informal settlements, developing communities: water supply and sanitation. The Water Research Commission (WRC), WRC Report. 2008(1656/1):08. Available at https://www.wrc.org.za/Knowledge%20Hub%20Documents/.../1656-1-08.pdf.
- 44.Mosler H-J. A systematic approach to behavior change interventions for the water and sanitation sector in developing countries: a conceptual model, a review, and a guideline. Int J Environ Health Res. 2012;22(5):431–449. doi: 10.1080/09603123.2011.650156. [DOI] [PubMed] [Google Scholar]
- 45.Pickering AJ, Djebbari H, Lopez C, Coulibaly M, Alzua ML. Effect of a community-led sanitation intervention on child diarrhoea and child growth in rural Mali: a cluster-randomised controlled trial. Lancet Glob Health. 2015;3(11):e701–ee11. doi: 10.1016/S2214-109X(15)00144-8. [DOI] [PubMed] [Google Scholar]
- 46.Patil SR, Arnold BF, Salvatore AL, Briceno B, Ganguly S, Colford JM, Jr, et al. The effect of India’s total sanitation campaign on defecation behaviors and child health in rural Madhya Pradesh: a cluster randomized controlled trial. PLoS Med. 2014;11(8):e1001709. doi: 10.1371/journal.pmed.1001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gebresilase Y. Community led total sanitation and empowerment: the case of Dorze Hyzo community, SNNP Region of Ethiopia (a phenomenological study) OIDA Int J Sustain Dev. 2010;01(09):99–107. [Google Scholar]
- 48.De Buck E, Van Remoortel H, Hannes K, Govender T, Naidoo S, Avau B, et al. Approaches to promote handwashing and sanitation behaviour change in low-and middle income countries: a mixed method systematic review. Campbell Syst Rev. 2017;7:1–447. [Google Scholar]
- 49.Galvin M. Talking shit: is community-led total sanitation a radical and revolutionary approach to sanitation. Wiley Interdiscip Rev Water. 2015;2(1):9–20. [Google Scholar]
- 50.Whaley L, Webster J. The effectiveness and sustainability of two demand-driven sanitation and hygiene approaches in Zimbabwe. J Water Sanit Hyg Dev. 2011;1(1):20–36. [Google Scholar]
- 51.Hahn RA, Truman BI. Education improves public health and promotes health equity. Int J Health Serv. 2015;45(4):657–678. doi: 10.1177/0020731415585986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon requesting the primary author.