Proc. R. Soc. B 285, 20172456 (Published 21 March 2018) (doi:10.1098/rspb.2017.2456)
1. Purpose
There were two errors in our original submission: (i) an error in the equation used to calculate egested nitrogen, ultimately leading to a small (1.8%) reduction in our estimate of nitrogen egestion; and (ii) an omitted step in converting wet weight of ingested material to dry mass, leading to a further approximately 50% reduction in the estimate of nitrogen egestion at the individual and population level.
In the nitrogen egestion equation, N was used to define two different variables, and the second N was incorrectly defined and assessed as the percentage concentration of nitrogen found in the grey reef shark tissue, for which we used 14.84% (unpublished data from McCauley et al. [5]). We have rewritten the equation (below) and updated the relevant term to correctly represent the per cent of nitrogen found in the prey species, for which we used 13.0% for the Pacific mackerel (Scomber japonicus) [4]. The rate of nitrogen egested by an individual i grey reef shark per day (EN) was defined as
| 1 |
where I, the mass ingested by the grey reef shark (kg d−1), is 2% of the total body weight of the individual shark [7], β, the assimilation efficiency, is 76% [8], Nprey, the mean percentage concentration of nitrogen in the tissue of prey species, is 13.0% nitrogen [4]. The upper bound of total nitrogen egested (kg) by an individual shark onto the nearshore habitat was then calculated as the product of EN and the number of days the shark was detected on the acoustic array (located on the forereef) over the course of the 4 year study (mean = 345.8 ± 60.7 days). To estimate the upper bound of nitrogen egested by the entire grey reef shark population at Palmyra per day, we took the average length of a male (138.7 cm) and female (146 cm) grey reef shark from Bradley et al. [2], and the total population and sex ratio estimates from Bradley et al. [3].
In the original submission, I was incorrectly reported in wet weight. We now use the wet to dry weight conversion reported for tropical teleosts (not available for Carcharhinids) in Allgeier et al. [1] where
| 2 |
to convert kg wet weight x to kg dry weight y. Therefore, for a 20 kg shark, EN is calculated as the total consumption by the shark per day (2% of 20 kg d−1 = 0.4 kg d−1), converted to dry weight according to equation (2) (0.279 × 0.4 kg d−1 + 0.0789 = 0.19 kg d−1), multiplied by 1–0.76 where 76% is the assimilation efficiency ((1–0.76) × 0.19 kg d−1 = 0.046 kg d−1), multiplied by the 13% mean percentage concentration of nitrogen in the prey tissue (0.13 nitrogen × 0.046 kg d−1), such that total nitrogen egested for that individual is equal to 0.006 kg nitrogen d−1. If that same shark was detected on the acoustic array a total of 100 days during the course of the 4 year study, then the upper bound of total nitrogen egested on to the forereef during the study was calculated as 0.006 kg nitrogen d−1 × 100 days = 0.6 kg nitrogen.
2. Results
Corrected estimates are as follows. On average, tagged female sharks egested as much as 6.65 ± 0.32 g nitrogen d−1, and tagged male sharks egested as much as 5.58 ± 0.26 g nitrogen d−1. Accounting for detections on the forereef during the 4 year duration of the study, tagged female (n = 27) and male (n = 13) grey reef sharks are estimated to have egested as much as 62.45 and 23.43 kg nitrogen, respectively, across the atoll and near-shore ecosystem. The maximum potential biomass subsidy from pelagic resources was 73.9 kg nitrogen transported onto Palmyra Atoll reefs by the tagged individuals over the study duration. An average female and male individual from the population was estimated to egest as much as 6.47 and 5.64 g d−1 of pelagic nitrogen in Palmyra Atoll, respectively. Extrapolating to the total population, we then estimate an upper bound of total biomass transfer of 50.94 kg nitrogen d−1, of which as much as 43.81 kg nitrogen d−1 is a subsidy from pelagic resources brought to the reef by grey reef sharks.
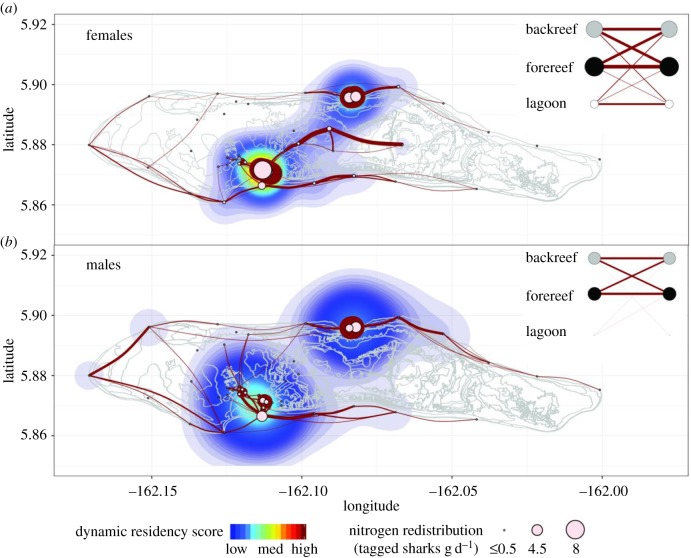
Figure 3.
(corrected) The four-year movement networks of (a) female (n = 28) and (b) male (n = 13) grey reef sharks overlaid on kernel densities that represent dynamic residency at each receiver. Networks include only movements that took less than or equal to 110 h and have been filtered to show the 75 most frequently used routes by each sex. Edge thickness represents the frequency of movements (M range = 36–2711; F range = 129–13 131). The dynamic residency score was calculated as the node strength (Si) of each receiver divided by 100 and multiplied by a standard residency index, R (M range = 1–1401; F range = 2–10 474). The size of each node represents the potential nitrogen redistribution by the tagged grey reef sharks (see table 2). The inset habitat networks illustrate the relative frequency of shark movements within and between geographical zones, with the size of the nodes representing the relative detection frequencies in each habitat; the left-hand nodes represent the zone the sharks moved into after last being detected in the habitat depicted by the right-hand node. The base map of Palmyra Atoll was acquired from NOAA [6].
Table 2.
(corrected) The five nodes around which the greatest quantity of nitrogen (N) is estimated to have been distributed by the tagged male or female grey reef sharks per day, based on the dynamic residency score of each node. See figure 1 (in original paper) for location of nodes.
| acoustic receiver (geographical zonea) | node strength | residency index (%) | dynamic residency score | quantity of nitrogen potentially distributed by the tagged grey reef sharks (g d−1) |
|---|---|---|---|---|
| females | ||||
| 18 (FR) | 11 674 | 89.73 | 10 474.62 | 8.22 |
| 40 (BR) | 9023 | 81.23 | 7329.64 | 5.75 |
| 16 (FR) | 7360 | 84.11 | 6190.47 | 4.86 |
| 10 (FR) | 7122 | 79.66 | 5673.21 | 4.45 |
| 60 (FR) | 4094 | 92.19 | 3774.33 | 2.96 |
| males | ||||
| 16 (FR) | 1704 | 82.26 | 1401.72 | 2.92 |
| 60 (FR) | 1702 | 75.55 | 1285.83 | 2.67 |
| 10 (FR) | 1567 | 55.34 | 867.22 | 1.80 |
| 18 (FR) | 1727 | 39.11 | 675.43 | 1.40 |
| 40 (BR) | 1413 | 32.26 | 455.84 | 0.95 |
aGeographical zones include the fore-reef (FR), back-reef (BR) and lagoon.
3. Discussion
Correcting our reported nitrogen egestion estimates by 54% for the entire grey reef shark population at Palmyra (52% for female sharks in the study; 56% for male sharks in the study) does not change the proportions of nitrogen distributed across the movement network, nor does it change our conclusion: reef sharks transfer a significant amount of nitrogen to and within an isolated atoll. The total Palmyra shark population heterogeneously egests as much as 50.9 kg d−1 (not 94.5 kg d−1 as was originally reported) of nitrogen around the reefs, approximately 86%, or 43.8 kg nitrogen d−1 (not 81.3 kg nitrogen d−1 as was originally reported), of which is likely derived from pelagic resources. We note that our nitrogen flux estimate is for egestion only and does not account for excretion; as a result, we are underestimating total nitrogen flux by the grey reef shark.
Acknowledgments
We would like to thank Jacob Allgeier for pointing out these errors in our original submission, and for working with us to correct them.
References
- 1.Allgeier JE, Wenger SJ, Rosemond AD, Schindler DE, Layman CA. 2015. Metabolic theory and taxonomic identity predict nutrient recycling in a diverse food web. Proc. Natl Acad. Sci. USA 112, E2640–E2647. ( 10.1073/pnas.1420819112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley D, Conklin E, Papastamatiou YP, McCauley DJ, Pollock K, Kendall BE, Gaines SD, Caselle JE. 2017. Growth and life history variability of the grey reef shark (Carcharhinus amblyrhynchos) across its range. PLoS ONE 12, e0172370 ( 10.1371/journal.pone.0172370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley D, Conklin E, Papastamatiou YP, McCauley DJ, Pollock K, Pollock A, Kendall BE, Gaines SD, Caselle JE. 2017. Resetting predator baselines in coral reef ecosystems. Sci. Rep. 7, 43131 ( 10.1038/srep43131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czamanski M, Nugraha A, Pondaven P, Lasbleiz M, Masson A, Caroff N, Bellail R, Tréguer P. 2011. Carbon, nitrogen and phosphorus elemental stoichiometry in aquacultured and wild-caught fish and consequences for pelagic nutrient dynamics. Mar. Biol. 158, 2847 ( 10.1007/s00227-011-1783-7) [DOI] [Google Scholar]
- 5.McCauley DJ, Young HS, Dunbar RB, Estes JA, Semmens BX, Micheli F. 2012. Assessing the effects of large mobile predators on ecosystem connectivity. Ecol. Appl. 22, 1711–1717. ( 10.1890/11-1653.1) [DOI] [PubMed] [Google Scholar]
- 6.NOAA National Ocean Service, National Centers for Coastal Ocean Science. 2016. Project details. Benthic habitat mapping of Palmyra Atoll. See https://coastalscience.noaa.gov/projects/detail?key=70 (accessed on 1 May 2016).
- 7.Wetherbee B, Cortés E, Bizzarro J. 2012. Food consumption and feeding habits. In Biology of sharks and their relatives (eds Carrier JC, Musick JA, Heithaus MR), pp. 239–264. Boca Raton, FL: CRC Press. [Google Scholar]
- 8.Wetherbee BM, Gruber SH. 1993. Absorption efficiency of the lemon shark Negaprion brevirostris at varying rates of energy intake. Copeia 1993, 416–425. ( 10.2307/1447140) [DOI] [Google Scholar]