Table 3.
Substrate scope of trifluoromethyl imines with β-substituted α, β-unsaturated N-acylpyrroles.[a]
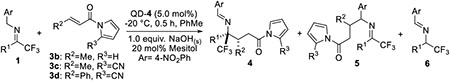 | ||||||
|---|---|---|---|---|---|---|
| entry | R1 | 3 | 4/5; 4/6[b] | d.r[b] | Yield of 4[c] | ee of 4 (%)[d] |
| 1 | Me; 1A | 3b | --; -- | -- | -- | -- |
| 2 | Me; 1A | 3c | 85/15; >99/1 | >95/5 | 72 (4Ac) | 87 |
| 3 | Et; 1B | 3c | 76/24; >99/1 | >95/5 | 55(4Bc) | 81 |
| 4[e] | H; 1J | 3c | 95/5; 85/15 | >95/5 | 73 (4JC) | 85 |
| 5[f] | H; 1J | 3d | 95/5; 80/20 | >95/5 | 52 (4Jd) | 80 |
Unless noted, reactions were performed with 1 (0.2 mmol), 3 (0.4 mmol), mesitol (20 mol%) and anhydrous NaOH solid powder (1.0 equiv.) in PhMe (2.0 mL) with catalyst QD-4 (5.0 mol%) at −20 °C.
Determined by 19F NMR analysis.
Isolated yield of product.
Determined by HPLC analysis.
The absolute configuration of 4Jc was determined to be (S, S) by X-ray crystallographic analysis.[20]
PhMe/Ch2Ch2 (4/1, totally 2.0 mL) was used as solvent.
