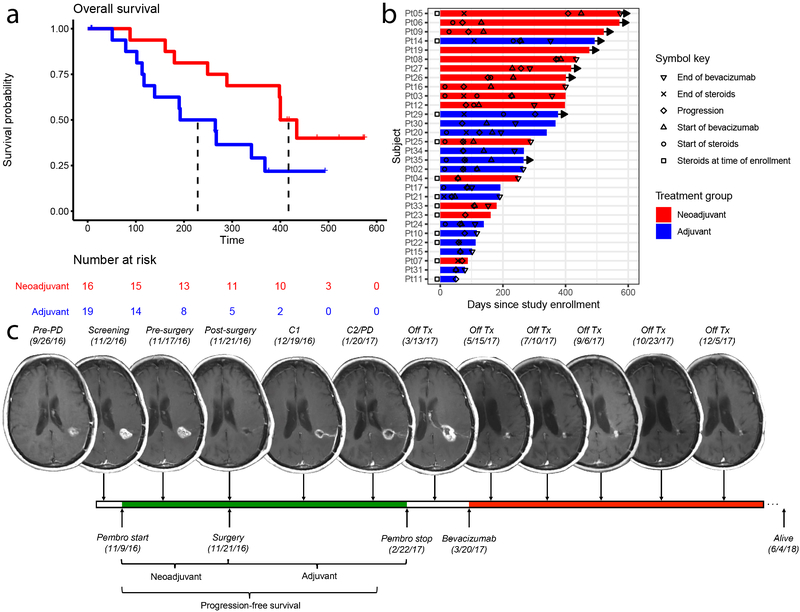
Figure 1. Neoadjuvant pembrolizumab confers significant improvement in overall and progression-free survival in patients with recurrent glioblastoma.
Patients in the neoadjuvant arm (red) received 200 mg pembrolizumab 14±5 days prior to surgical resection; patients in the adjuvant-only arm (blue) did not; both groups received 200 mg adjuvant pembrolizumab every 3 weeks. (a) Kaplan-Meier plot of overall survival. Median overall survival for the patients receiving adjuvant treatment only was 228.5 days, whereas median survival in the neoadjuvant group was 417 days (hazard ratio (HR) 0.39 neoadjuvant/adjuvant; P = 0.04 by log-rank test, n = 35 patients). (b) Swimmer plot of individual patients depicting start and stop times of dexamethasone or equivalent (as recorded at study MRI visit) and/or bevacizumab, if applicable, as well as time to progression, in days after enrollment. Bars represent overall survival in days. Rightward arrow indicates that the patient was still alive at the time of final data collection. (c) Serial magnetic resonance imaging (MRI) scans of a representative patient from the neoadjuvant group. The patient, a 66-year old female in first recurrence, is IDH negative and MGMT unmethylated. IDH: isocitrate dehydrogenase. MGMT: O6-methylguanine DNA methyltransferase. PD: progression of disease. Pembro: pembrolizumab. Tx: treatment.