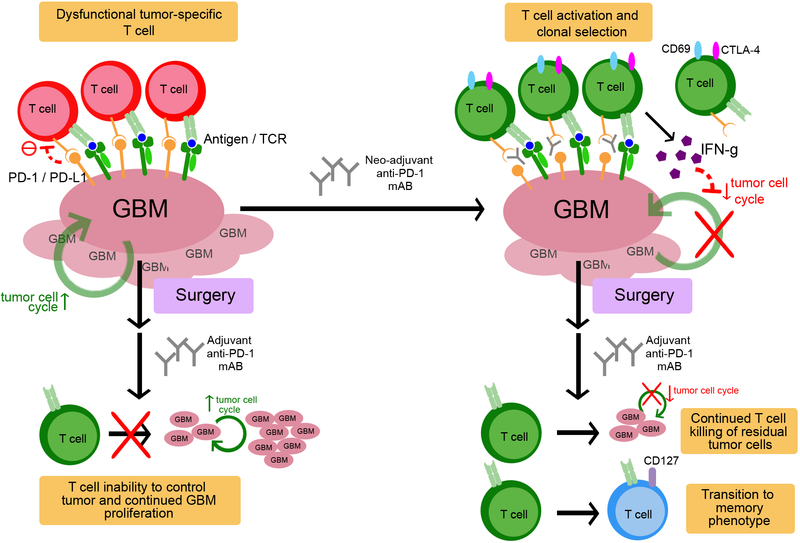
Figure 5. Proposed mechanism of neoadjuvant PD-1 blockade in recurrent GBM.
Tumor infiltrating lymphocytes, if present, are rendered ineffective through the PD-1/PD-L1 axis. Neoadjuvant PD-1 blockade releases this checkpoint, enabling modulation of the T cell receptor (TCR) clonotypes with systemic activation and clonal selection of tumor-specific T cells. Such T cell activation in turn upregulates interferon-γ-related signaling, while downregulating tumor cell cycle related genes. After surgery, and with continued adjuvant anti-PD-1 monoclonal antibody administration, tumor-specific T cells continue to eliminate residual tumor cells and begin transitioning toward a T memory phenotype. In the adjuvant-only group, surgery occurs before checkpoint blockade release. Because of the reduced residual antigenic burden, TCR modulation is less robust and fewer tumor-specific T cells are activated. With fewer tumor-specific T cells, the remaining tumor cells are able to proliferate at a more rapid pace.