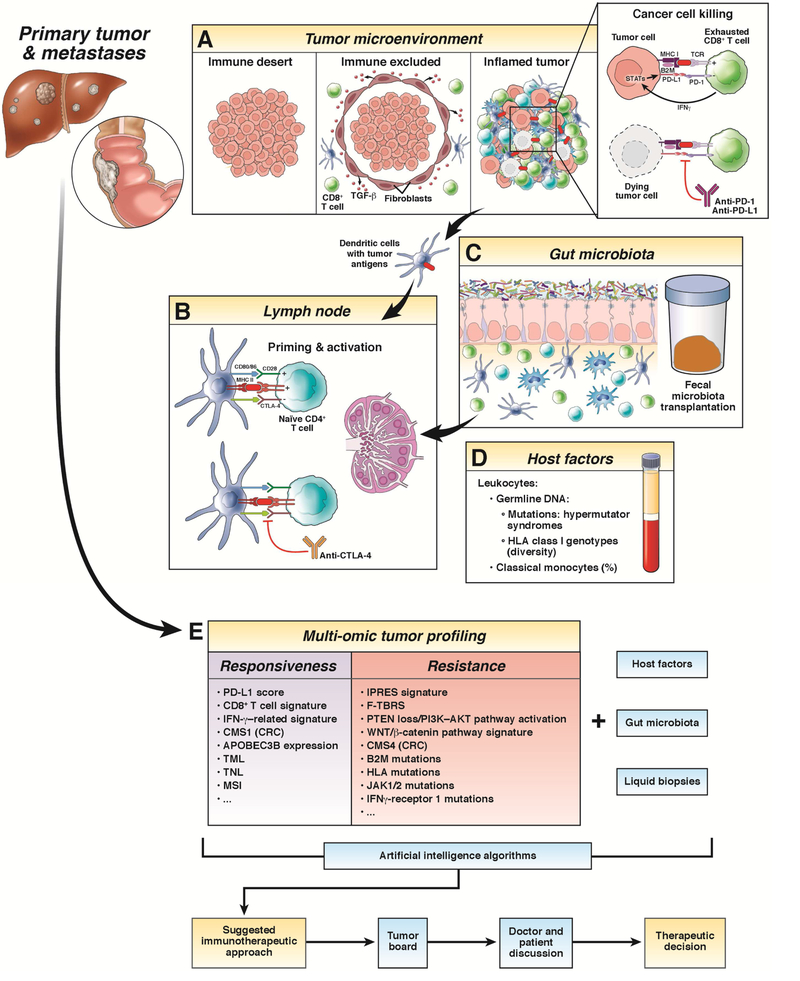
Figure 2:
This illustration provides a schematic and general overview of the different and most relevant mechanisms correlated with the efficacy of immune checkpoint inhibitors (ICIs). (a) There are three possible types of tumor microenvironment (TME) based on the presence of immune cells and their interplay with tumor cells: immune desert TME (characterized by the immunologic ignorance of tumor cells), immune excluded TME (characterized by the presence of immune cells in the tumor margin, due to the effect, among others, of TGF-β secreted by cancer-associated fibroblasts) and inflamed TME (characterized by the T-cell exhaustion in the context of immunosuppressive conditions). Expression of programmed death-ligand 1 (PD-L1) on the surface of tumor cells can be either constitutive by oncogenic activation (innate immune resistance) or induced by surrounding immune cells (adaptive immune resistance). Blockade of the programmed cell death 1 (PD-1)/PD-L1 interaction reverses T cell exhaustion and enables tumor cell killing. (b) Lymph nodes are where antigen-presenting cells, such as dendritic cells, participate in the priming and activation of naive or resting T cells. Cytotoxic Tlymphocyte antigen 4 (CTLA-4) expression is induced in the T cell surface at the time of the initial response to the tumor antigen (presented by the MHC II) to downregulate the response amplitude. CTLA-4 blockade interrupts its interaction with CD80 or CD86, avoiding attenuation of T cell stimulation. (c) The influence of different commensal bacteria of the intestinal microbiome (Faecalibacterium spp., A. muciniphilia, E. hirae, Bacteroides spp., and Bifidobacteria spp.) together with (d) several host factors, such as the percentage of classical monocytes or diversity in the HLA class I molecules, have been recently associated with ICI therapy outcomes. (e) Besides the multi-omic tumor profiling, which permits static evaluation of different molecular features associated with the efficacy of ICI, liquid biopsies can be considered a complement that offers spatial and temporal dynamic information. Integration of all these aspects using artificial intelligence algorithms, together with meticulous evaluation by a tumor board, and always considering patient’s desires, will help in the therapeutic decisionmaking process. Abbreviations: CMS, consensus molecular subtype; CRC, colorectal cancer; TML, tumor mutation load; TNL, tumor neoantigen load; MSI, microsatellite instability; IPRES, innate anti-PD-1 resistance; F-TBRS, pan-fibroblast TGF-β response signature.