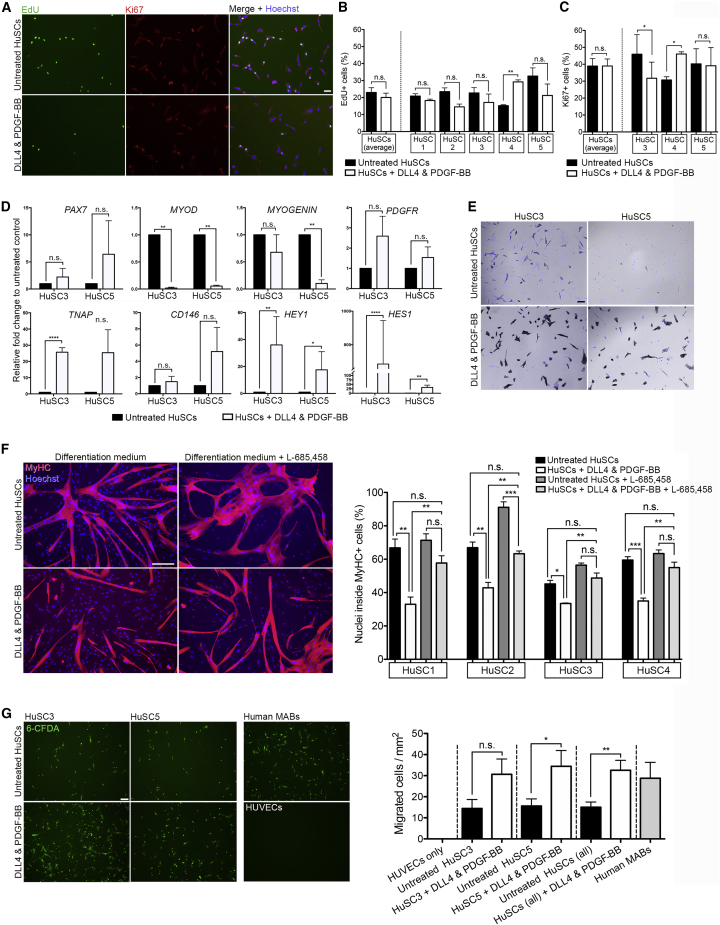
Figure 5.
Effect of DLL4 and PDGF-BB Treatment on Human SC-Derived Myoblasts
(A) Representative images of CD56+ FACS-purified human SC-derived myoblasts from two individuals (HuSC 3 and 5) expanded in control conditions (upper panel) or treated with DLL4 and PDGF-BB (lower panel) for 1 week, then pulsed for 2 h with EdU (green) and co-immunostained with Ki67 (red).
(B) Quantification of the percentage of EdU+ cells (N = 5).
(C) Quantification of Ki67+ cells (N = 3).
(D) Real-time qPCR analysis of myogenic (PAX7, MYOD, and MYOGENIN), perivascular (PDGFRB, TNAP, and CD146) and Notch signaling (HES1 and HEY1) genes in response to DLL4 and PDGF-BB treatment in HuSC 3 and 5 (n = 3 per line). Graphs show fold change to control conditions, statistical significance based on ΔCt.
(E) Phase contrast images of AP enzymatic activity (violet) and Hoechst (blue) in untreated and treated HuSCs.
(F) Differentiation of HuSCs in response to DLL4 and PDGF-BB treatment and Notch inhibition. Cells expanded in control or treatment conditions for 1 week and induced to differentiate for 4 days in the presence or absence of L-685,458. Pictures representatives from HuSC1, and graphs quantify the percentage of MyHC+ cells (N = 4).
(G) Representative images of the lower chambers of transwell assays. Untreated/treated HuSCs and human muscle pericyte-derived mesoangioblasts (MABs; technical control) were seeded for 8 h on HUVECs. Right graph: number of 6-CFDA+ migrated cell/mm2 in each condition. N = 3; means ± SEM.
Statistical significance based on paired Student's t test (B, C and cell-line-specific data in G), unpaired Student's t test (D and pooled data in G) or one-way ANOVA with Tukey's multiple comparison (F). ∗p < 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001; n.s., not significant. Scale bars, 50 μm (A), 75 μm (F), and 100 μm (E and G).