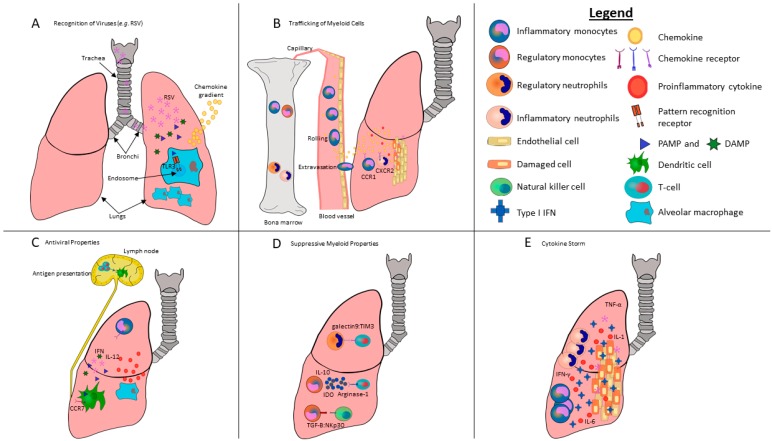
Figure 1.
Schematic of myeloid cells highlighting their ability to respond to pulmonary viral infections via the initiation and modulation of anti-viral inflammatory activity. Lung-resident myeloid cells, such as alveolar macrophages, utilize a complex sensory system to integrate disturbances of pulmonary tissues by viruses such as respiratory syncytial virus (RSV) into the activation of local effector leukocytes. (A) RSV enters and infects the lungs. Viral pathogen-associated molecular patterns (PAMPs), such as double-stranded RNA or danger associated molecular patterns (DAMPs), are detected by pattern recognition receptors (PRRs) in or on sentinel cells in the lungs, such as TLR3 in the endosomes of lung-resident macrophages. TLR stimulation activates the NF-κß signaling cascade, resulting in the release of chemokines and inflammatory cytokines. A chemokine gradient forms between the lungs and bone marrow. (B) Homeostatic bone marrow tends to retain CXCR4+ neutrophils and monocytes through endogenous expression of high levels of CXCL12. However, the release of PAMPs, as well as the secretion of cytokines and chemokines as a consequence of pulmonary RSV infections, is sensed by cells in the bone marrow, which in turn allow recruitment of new neutrophils and monocytes from the bone marrow into the lungs. Specifically, G-CSF downregulates CXCR4 on neutrophils, triggering their release. Similarly, CCL2 is produced in the bone marrow by endothelial cells following TLR signaling in infected lungs, which is crucial for inflammatory monocyte release into the bloodstream. Once in the bloodstream, these cells sense disrupted endothelium from the viral infection, which triggers a complex adhesion cascade. Activated Ly6Chi inflammatory monocytes are recruited to the site of infection by a variety of chemokine receptors including CCR1, 5 and 6, as well as CXCR2 binding to their respective ligands. (C) Once at the site of infection, they differentiate into dendritic cells and macrophages that initiate an inflammatory cascade that includes copious amounts of inflammatory cytokines, in particular IL-12 and IFN-γ, which are potent inducers of Th1-biased immune responses. Once these dendritic cells and macrophages acquire viral antigens, they home to lymph nodes via chemokine receptors, including CCR7. Monocyte-derived dendritic cells that home to lymph nodes present viral antigens to naïve CD4+ and CD8+ T-cells that are required to kill infected cells. (D) The basic neutrophil function of clearing an inflamed area by removing killed pathogens and host cells contributes to reduced inflammation and wound debridement. Neutrophils are also capable of promoting tissue repair and increased angiogenesis. Further, monocytes can suppress lymphocytes in various clinical scenarios. In lungs, myeloid cells are able to inhibit pro-inflammatory tissue-resident leukocytes through direct cell-to-cell contact through galectin9/TIM3 and the effect of TGF-β on NKp30 in order to regulate T-cells and NK cells, respectively. Myeloid cells can also exert suppressive functions through secretion of soluble factors such as IL-10, arginase-1 and indoleamine 2,3-dioxygenase. (E) We speculate that disruption of the cellular sensing of type I IFN responses can result in excessive production of pro-inflammatory cytokines, including IFN-γ, IL-1, IL-6, and TNF-α, leading to a toxic cytokine storm. The fatal outcome of severe lung infections is shown to be correlated with the early persistent production of inflammatory cytokines and chemokines that recruit neutrophils and monocytes. While inflammatory cytokines and chemokines are essential for effective control of viral infections, they can also contribute to the severity of disease and tissue damage.