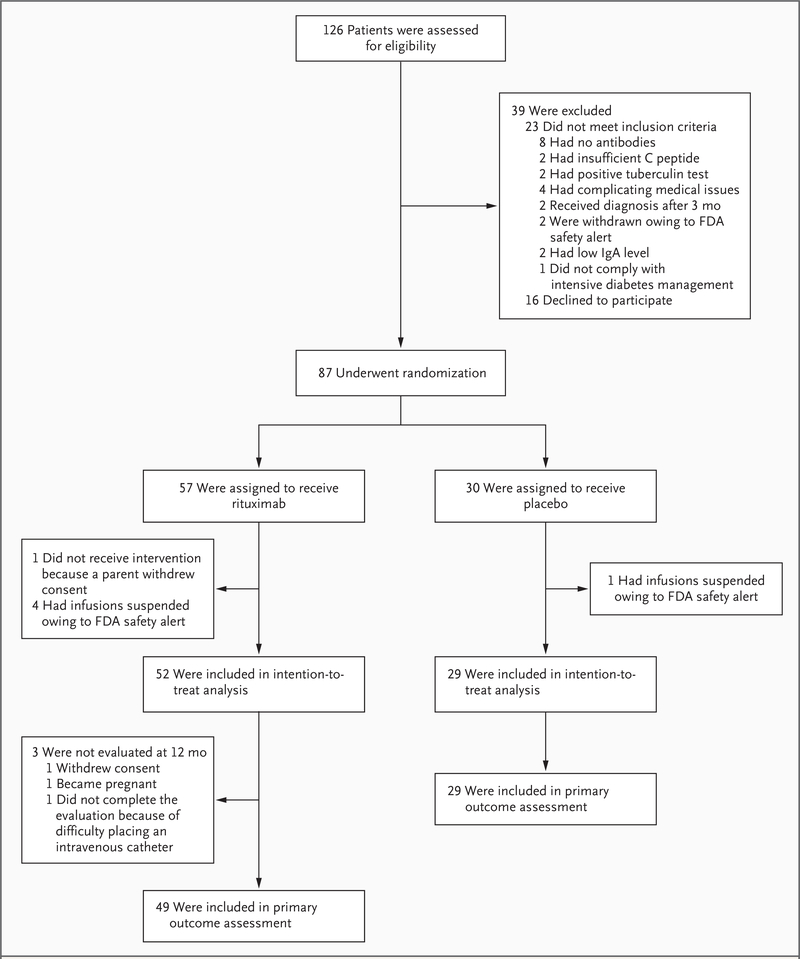
Figure 1. Enrollment, Randomization, and Follow-up of Study Participants.
Between May 2006 and August 2007, a total of 126 patients were screened and 87 underwent randomization. Of the 87 patients who underwent randomization, 81 composed the intention-to-treat cohort, of whom 78 contributed to the primary effectiveness analyses at 12 months. In 5 of the 6 patients who were excluded, infusions were stopped after an FDA safety alert concerning the development of progressive multifocal leukoencephalopathy in other study populations receiving rituximab; in the other patient, parental consent was withdrawn before administration of the initial infusion.