Table 1.
Substrate Scope of α-Quaternary Cyclic Hydroxamic Acid Derivativesa
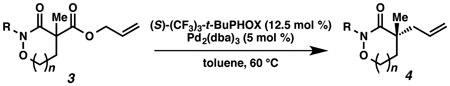 | ||||
|---|---|---|---|---|
| entry | substrate | R | yield (%)b | ee (%)c |
| 1 | 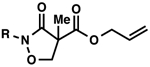 |
Bz (3a→4a)d | 98 | 73 |
| 2 | Boc (3b→4b) | 95 | 72 | |
| 3 | PhO(CO) (3c→4c) | 95 | 73 | |
| 4 | 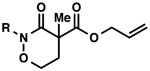 |
Bz (3d→4d) | 29 | 88 |
| 5 | Piv (3e→4e) | 48 | 73 | |
| 6 | Bn (3f→4f) | trace | ND | |
| 7 | Boc (3g→4g) | 67 | 85 | |
| 8 | Cbz (3h→4h) | 89 | 84 | |
| 9 | PhO(CO) (3i→4i) | 70 | 87 | |
| 10 | 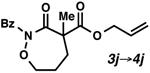 |
81 | 93 | |
Reaction performed under the conditions of Figure 3 at 60 °C.
All reported yields are for isolated products.
Enantiomeric excesses were determined by chiral SFC analysis.
Absolute configuration was assigned by VCD spectroscopy22 supported by theoretical calculations (see supporting information).
