Figure 13.
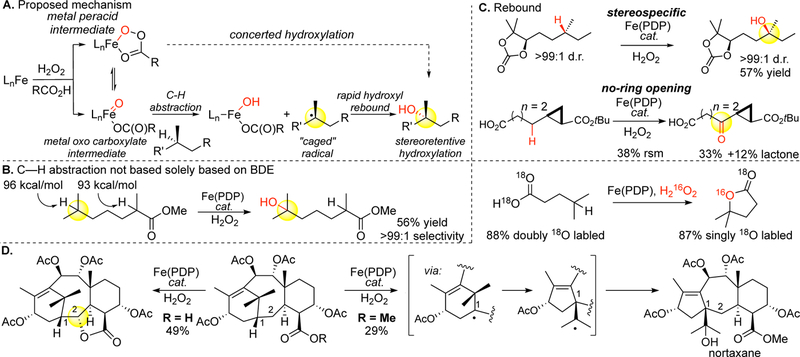
Mechanistic considerations with Fe(PDP) hydroxylations. (A) Proposed mechanism for Fe(PDP)-mediated C(sp3)–H hydroxylation. (B) The site-selectivity in C–H cleavage with the Fe(PDP) oxidant is not solely based on BDE. (C) Oxidations with Fe(PDP) do not scramble stereocenters or open cyclopropane rings, indicating that no long-lived carbon-centered radicals are formed. (D) Evidence for a stepwise mechanism is seen in Fe(PDP) oxidation of a taxane derivative where a ring-contracted nortaxane product is formed. Carboxylic acids on the substrate can direct oxidation away from the site that is intermolecularly favored with Fe(PDP). Collectively this suggests that Fe(PDP) oxidations proceed via a carboxylate-bound oxidant through a late, product-like C–H cleavage step and a rapid hydroxyl rebound step.
