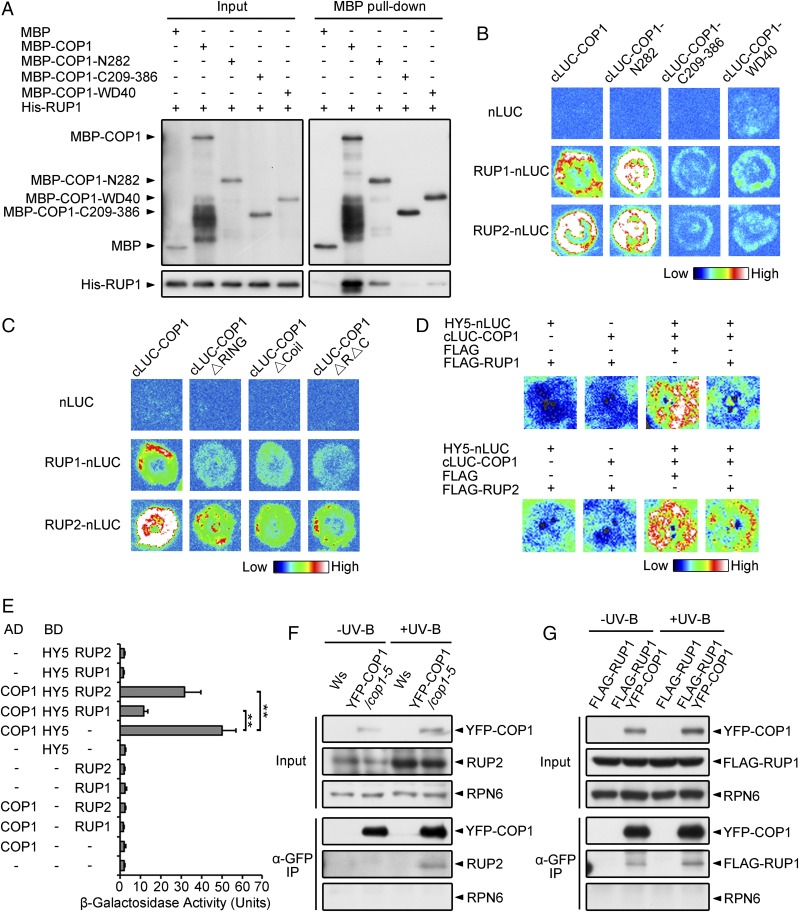
Fig. 4.
COP1 Interacts with RUP1 and RUP2. (A) RUP1 interacts with full-length and truncated COP1 in vitro. Purified MBP, MBP-COP1, MBP-COP1-N282, MBP-COP1-C209-386, or MBP-COP1-WD40 was incubated with His-RUP1 before being pulled down by Amylose Resin. His-RUP1 was detected by anti-RUP1 antibodies. (B) RUP1 and RUP2 interact with full-length and truncated COP1 in N. benthamiana, as assayed by firefly LCI. The color bar shows the range of luminescence intensity. (C) COP1 interacts with RUP1 and RUP2 primarily through the RING domain and Coil domain in N. benthamiana, as assayed by firefly LCI. The color bar shows the range of luminescence intensity. (D) RUP1/RUP2 impairs the COP1–HY5 interaction in N. benthamiana, as assayed by firefly LCI. The color bar below shows the range of luminescence intensity in each image. The minus symbols (−) indicate empty vectors of nLUC, cLUC, or FLAG. (E) RUP1/RUP2 impairs the COP1–HY5 interaction in yeast. β-Galactosidase activity was quantified using o-nitrophenyl-β-d-galactopyranoside as a substrate (mean ± SD, n = 3). The asterisks indicate significant differences by Student’s t test (**P < 0.01). (F) YFP-COP1 associates with RUP2 in Arabidopsis. Total proteins were extracted from 4-d-old seedlings grown under −UV-B and +UV-B light conditions for co-IP with Dynabeads Protein G and anti-GFP antibodies. Proteins were analyzed by immunoblotting with anti-GFP, anti-RUP2, and anti-RPN6 antibodies. RPN6 was used as a loading and negative control. (G) YFP-COP1 associates with FLAG-RUP1 in Arabidopsis. Total proteins were extracted from 4-d-old seedlings grown under −UV-B and +UV-B light conditions for co-IP with Dynabeads Protein G and anti-GFP antibodies. Proteins were analyzed by immunoblotting with anti-GFP, anti-FLAG, and anti-RPN6 antibodies. RPN6 was used as a loading and negative control.