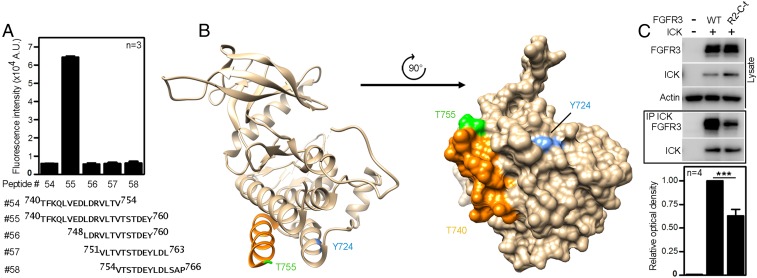
Fig. 3.
The 751VLTVTSTDEY760 motif in FGFR3 is required for the interaction with ICK. (A) Averaged fluorescence intensities from three replicates of the peptide microarray involving peptides from FGFR3 C-terminal region. (B) Ribbon and surface representations of the crystal structure of the TK domain of FGFR3 (PDB ID code 4K33). Residue Y724 and C-terminal region implicated in ICK binding by co-IP experiments and peptide microarray analysis are highlighted in blue and orange/green, respectively. Orange, α-helix; green, the residue T755 (unstructured). Note that the absence of structural information for residues 756STDEY760 is suggestive of structural disorder. (C) The putative ICK interacting motif on FGFR3 (751VLTVTSTDEY760) was replaced by the analogous sequence from FGFR2 (760ILTLTTNEEY769), creating the FGFR3-R2-C-t chimera. The co-IP of FGFR3-R2-C-t with ICK compared with wild-type FGFR3 (Student’s t test; ***P < 0.001).