Significance
Newborns show a remarkable ability to detect faces even minutes after birth, an ecologically fundamental skill that is instrumental for interacting with their conspecifics. What are the neural bases of this expertise? Using EEG and a slow oscillatory visual stimulation, we identified a reliable response specific to facelike patterns in newborns, for which underlying cortical sources overlap with the adult face-specific cortical circuit. This suggests that the development of face perception in infants might rely on an early cortical route specialized in face processing already shortly after birth.
Keywords: facelike pattern detection, human newborns, frequency tagging, EEG, face processing
Abstract
Humans are endowed with an exceptional ability for detecting faces, a competence that, in adults, is supported by a set of face-specific cortical patches. Human newborns, already shortly after birth, preferentially orient to faces, even when they are presented in the form of highly schematic geometrical patterns vs. perceptually equivalent nonfacelike stimuli. The neural substrates underlying this early preference are still largely unexplored. Is the adult face-specific cortical circuit already active at birth, or does its specialization develop slowly as a function of experience and/or maturation? We measured EEG responses in 1- to 4-day-old awake, attentive human newborns to schematic facelike patterns and nonfacelike control stimuli, visually presented with slow oscillatory “peekaboo” dynamics (0.8 Hz) in a frequency-tagging design. Despite the limited duration of newborns’ attention, reliable frequency-tagged responses could be estimated for each stimulus from the peak of the EEG power spectrum at the stimulation frequency. Upright facelike stimuli elicited a significantly stronger frequency-tagged response than inverted facelike controls in a large set of electrodes. Source reconstruction of the underlying cortical activity revealed the recruitment of a partially right-lateralized network comprising lateral occipitotemporal and medial parietal areas overlapping with the adult face-processing circuit. This result suggests that the cortical route specialized in face processing is already functional at birth.
As a highly social species, humans display a set of exceptional key competences for social interactions that include the ability to detect, recognize, and memorize faces and to associate them with emotions and intentions (1). In the adult brain, face-processing skills are coupled with a relatively highly face-specific set of cortical patches mainly localized in the ventrolateral occipitotemporal cortex, often bilaterally but more consistently present in the right hemisphere (2, 3), and also extending to parietal, frontal, and subcortical areas (4). Among those patches, the occipitotemporal ones appear arranged in a similar stereotypical pattern in humans, macaque monkeys (5), and even marmosets (6), suggesting a phylogenetic continuity in the primates’ neural systems underlying face processing.
Ontogenetically, a behavioral bias for faces is detected very early: Human newborns within an hour of birth show a behavioral preference for canonically oriented faces, even when they are presented in the form of highly schematic geometrical patterns (two squares on top of one square, symmetrically inserted in an oval contour), over other kinds of visually controlled nonfacelike stimuli (e.g., geometric patterns in which the configuration is incompatible with that of a face) (7–9). This early preference, observed both for schematic facelike configurations and real faces (10, 11), might already be present during the third trimester of pregnancy (12), and it is shared with other animal species like chicks and macaque monkeys (13–15). Preferential orientation to faces might be instrumental to increase newborns’ visual exposure to faces compared with other visual categories (16), providing the basis for rapidly developing specific face-processing skills.
What are the neural bases of this early bias for faces in the human baby brain? Is there a universally shared neural system that newborns deploy when processing faces vs. other kinds of stimuli? The earliest evidence available to date for an early neural response to faces comes from EEG and fMRI/PET studies in infants already 2 to 4 mo old. The EEG studies compared the event-related potentials (ERPs) evoked by canonically oriented faces vs. inverted faces (17, 18) or vs. noise images with equivalent low-level visual properties (19), or contrasted the response to novel vs. familiar faces (20); in all cases, faces elicit a higher amplitude of the N290 and/or P400 ERP waves at occipitotemporal electrodes. A recent study in 4- to 6-mo-old infants using a novel EEG frequency-tagging paradigm (see more below) alternative to ERPs, confirms these results by showing a clear response to faces (compared with objects/scenes) in right lateral occipitotemporal electrodes (21). Although none of these studies attempted to reconstruct the anatomical sources of the EEG effects, their results are broadly compatible with the occipitotemporal neural generators of specific face-processing ERP signals seen in adults (22). The only fMRI investigation on face-specific cortical responses in infants (23) confirms that the large-scale organization of face-selective regions in the adult is already present at 4 to 6 mo after birth (fusiform gyrus, lateral occipital cortex, superior temporal sulcus, and medial prefrontal cortex; see also ref. 24), although with a lower-category selectivity (such face-selective regions respond to objects much more in infants than in adults). These results are suggestive of an early cortical protoarchitecture that preferentially engages when stimulated with faces. However, given the fast development of the visual system during the first 3 mo (25), it remains an open question whether (and to what extent) the same occipitotemporal circuit involved in face processing in infants and adults is already active at birth—when newborns’ experience with faces is still extremely limited—or whether such specialization emerges only later as a function of experience and/or maturation.
Here, we aim to bridge this gap by investigating the electrophysiological correlates of processing facelike stimuli in awake, attentive human newborns of less than 96 h after birth. We presented newborns with schematic and canonically oriented facelike stimuli (upright faces) and, as controls, with an inverted version of the same stimuli (inverted faces) (8, 9). As an additional control, we also presented “scrambled” faces organized in a nonfacelike, top-heavy fashion (more elements in the upper part than in the lower part of the oval) to investigate a previously proposed hypothesis that the preference for upright faces at birth may be mainly determined by a general preference for stimuli in which geometrical organization is top heavy vs. bottom heavy (26).
To comply with the extremely short duration of focused attention in newborns (27), we took advantage of a frequency-tagging paradigm—a design that “tags” the neural populations coding for a given stimulus by presenting that stimulus periodically at a specific (tag) temporal frequency and by measuring the neural response in the form of a sharp peak in the EEG power spectrum at the same frequency (28). Since both the EEG ongoing activity and EEG artifacts are broad band in frequency, the stimulus-related response in the frequency domain is easily discriminated from the stimulus-unrelated activity with relatively light artifact rejection, yielding a much higher signal-to-noise ratio (SNR) than the one obtained with ERPs. Oscillating visual stimulation based on the same principle has been widely used in the pioneer work on low-level visual function in newborns (e.g., refs. 29 and 30).
We used a high-density (125 electrodes) EEG system with a cap specifically designed for newborns (Electrical Geodesic, Inc.) to record EEG activity in 1- to 4-d-old healthy human newborns while presenting them with streams of schematic upright, inverted, and scrambled faces (Fig. 1) presented periodically at a frequency of 0.8 Hz. Newborns’ stimulus-related brain responses were quantified from the peaks of the EEG power spectrum at the frequency of stimulus presentation.
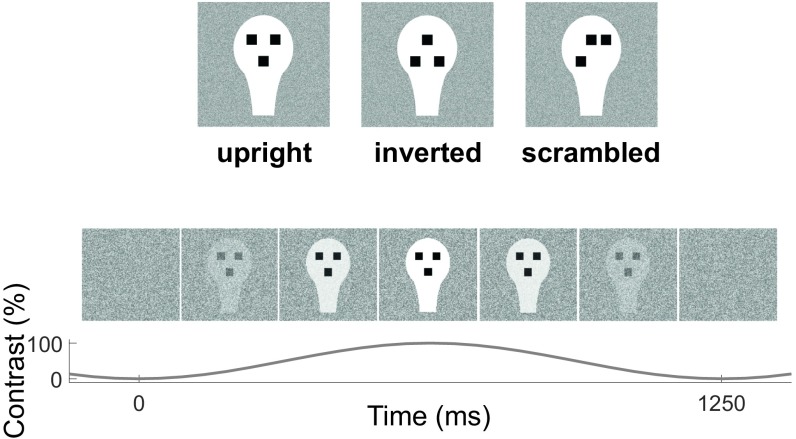
Fig. 1.
Visual stimulation. (Top) Stimuli used (upright, inverted, and scrambled faces). (Bottom) Illustration of one cycle of visual presentation with upright faces. Stimuli were presented dynamically with sinusoidal contrast modulation (0 to 100%) at a rate of 0.8 Hz (1 cycle = 1.25 s), overlapped onto a weakly contrasted background. Stimuli of the same type were presented continuously in blocks of 40 cycles (50 s) or until the subject stopped fixating.
Recent empirical and simulation studies showed that the newborn-scalp EEG topography is much less spatially smeared compared with that of the adult [the spatial decay of focal transients in newborn EEG signals is approximately three times steeper than the corresponding decay in adult EEG recordings (31)]. This property is generally attributed to the significantly thinner skull bones of the newborn. These studies suggest that by using a high spatial sampling of the scalp EEG and a realistic newborn head model and conductivity values (more than 1 order of magnitude higher than the adult ones) (31, 32), we could compute a reliable source reconstruction of the newborn’s EEG (33, 34). With these indications, taking advantage of high-density EEG recordings, we estimated the cortical generators of the scalp-level effects with a source localization model based on newborn’s realistic anatomical structure and electrical properties (34).
Results
Visual stimuli (upright, inverted, and scrambled geometric representations of faces, see Fig. 1) were presented dynamically with sinusoidal contrast modulation (0 to 100%) in blocks of 50 s (or until the subject stopped fixating) at a rate of 0.8 Hz (1 cycle = 1.25 s), overlapped onto a weakly contrasted dynamic white-noise background to minimize after-image effects. Data from the 10 subjects completing the protocol for all conditions were epoched on the basis of fixation intervals. After artifact rejection, the duration of clean EEG data per condition was, on average, 36.4 s (upright, 35.6 ± 17.6 s; inverted, 33.7 ± 13.7 s; scrambled, 39.9 ± 21.0 s), with no statistical difference among the three conditions [F(2,18) = 0.28, P = 0.68].
All Stimuli Elicit a Frequency-Tagged EEG Response.
We first tested whether with such short data intervals we could reliably measure a significant oscillatory response at the frequency of stimulation. Given the steep -like profile of the power spectrum in the low-frequency range of the stimulation frequency in newborns (35), we estimated the stimulus-unrelated “background” power at the tag frequency by a power-law fit of the power spectrum at neighboring frequency bins (±0.3 Hz). We then investigated the presence of a frequency-tagged response (FTR) by testing whether (and for which) electrodes the power at the tag frequency was significantly higher than the estimated background power. Statistical testing for this and for all of the following analyses was performed with a permutation-based nonparametric algorithm that tests the effects on the whole set of electrodes with no prior region-of-interest selection; the issue of multiple comparison is overcome by directly assessing the statistical significance on spatial clusters of channels (ref. 36; see Materials and Methods).
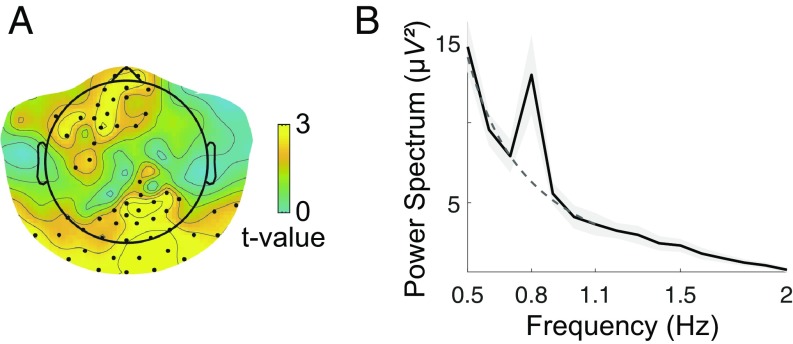
The results showed that at the tag frequency, the oscillating stimuli (all conditions merged) elicited a significantly higher power than the estimated background power in a large set of posterior electrodes (Pcorr < 0.003) and in a smaller frontal cluster (Pcorr < 0.022) (Fig. 2A). Visualization of the power spectrum in the posterior cluster shows that, as expected, this effect is due to a high peak of power at the tag frequency emerging from a -like profile at neighboring frequency bins (Fig. 2B).
Fig. 2.
FTR, all conditions merged. (A) Statistical map (one-tailed t test, corrected) of the difference between the power spectrum at the tag frequency (0.8 Hz) and the background power at the same frequency, estimated by a power-law fit of the power spectrum from the six neighboring frequency bins (±0.3 Hz). Electrodes belonging to a statistically significant cluster are marked with a black dot. Two clusters emerge: a posterior one (Pcorr < 0.003) and a frontal one (Pcorr < 0.022). (B) Power spectrum averaged over electrodes belonging to the posterior cluster (with P < 0.01) (black line) ± SEM (gray shadow) across subjects: although the overall frequency profile is well described by a power law (dashed dark-gray line, fitted in the interval 0.5 to 1.1 Hz), a peak neatly emerges at the tag frequency.
When conditions were considered separately, all stimuli elicited a significant peak at the tag frequency in a posterior cluster (upright, Pcorr < 0.004; inverted, Pcorr < 0.024; scrambled, Pcorr < 0.020), while only upright stimuli gave rise to an additional peak in a frontal cluster (Pcorr < 0.013) (SI Appendix, Fig. S1).
The Electrophysiological Signature of Facelike Pattern Processing.
Legitimated by the previous analyses, we quantified the FTR to each kind of stimulus as the ratio between the amplitude of the power spectrum at the tag frequency and the background power at the same frequency, estimated by the power-law fit as above.
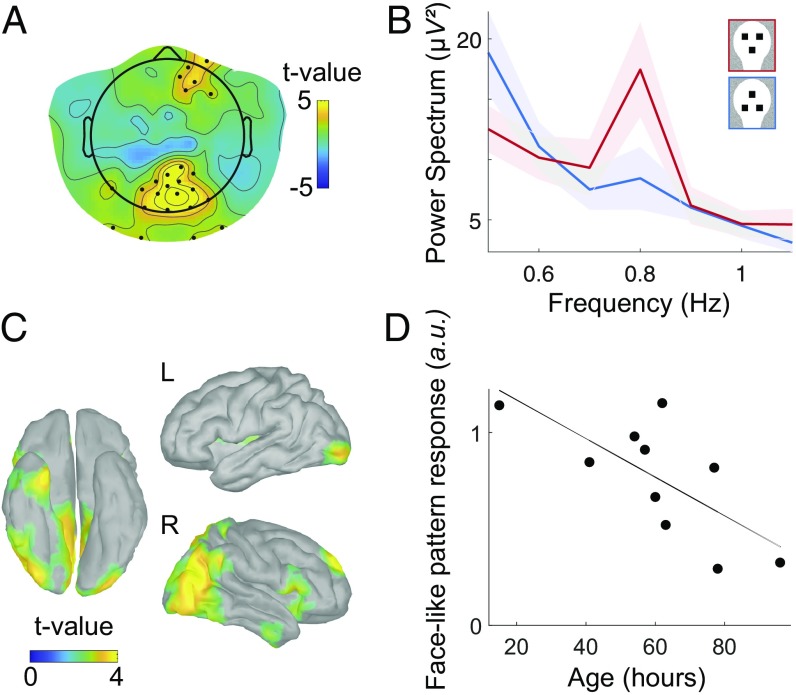
With that measure in hand, we moved to the direct investigation of the main focus of our research: characterizing the electrophysiological signature of processing facelike patterns by statistically comparing the FTR to facelike patterns, first to inverted and then to scrambled patterns. Compared with inverted faces, faces elicited a stronger FTR (Fig. 3B) in a wide posterior, slightly right-lateralized cluster (Pcorr < 0.003) (hereafter indicated as the main cluster) and in an anterior right-lateralized cluster (a weaker but significant effect; Pcorr < 0.049) (Fig. 3A). Remarkably, the effect in the main cluster was very robust (effect size: d = 2.49), consistently present in every single newborn (Fig. 4B) and independent from looking-time differences (correlation coefficient between the difference of the response to upright vs. inverted faces and the corresponding difference in looking times, R = 0.09, P = 0.8). In the following, we denote facelike pattern response as the difference between the response to upright faces and the response to inverted faces.
Fig. 3.
Comparison between upright vs. inverted faces. (A) Statistical map (t test, corrected) of the difference between the FTR to upright vs. inverted faces. Electrodes belonging to a statistically significant cluster are marked with a black dot. Response to faces is significantly stronger in posterior (Pcorr < 0.003) and right frontal (Pcorr < 0.049) clusters of electrodes. (B) Power spectrum averaged over the posterior cluster (channels with P < 0.01) for the two conditions (shaded contour indicates the SEM across subjects): the tag frequency peak for upright faces is clearly higher than the one for inverted faces. (C) Statistical map of the comparison of upright vs. inverted faces at the source level (P < 0.05, uncorrected), revealing a right-lateralized network that partly overlaps with the adult face-processing network. (D) Intersubject correlation between the facelike pattern response in the posterior cluster and the age from birth (R = 0.71, P < 0.02).
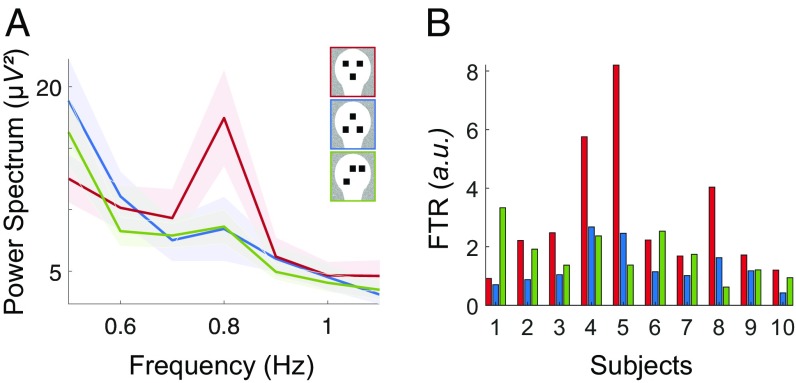
Fig. 4.
Response to scrambled faces is intermediate. (A) Power spectrum averaged over the main cluster associated to the facelike pattern effect (channels with P < 0.01), for the three conditions (shaded contour indicates the SEM across subjects): the average response to scrambled faces is more similar to the response to inverted faces. (B) Single-subject FTR in the main cluster for the three conditions. Although FTR(upright) > FTR(inverted) for each subject (reflecting the highly significant statistical difference), the FTR to scrambled faces is closer to the FTR to upright faces than it is to inverted faces in 4 of 10 subjects, suggesting an intermediate response.
The Effect of Age on the Facelike Pattern Response.
To test the impact of age/exposure to faces on the facelike pattern response, we performed a correlation between age (in hours after birth) and the average facelike pattern response in the main cluster. Results showed a significant negative correlation (R = 0.71, P < 0.02) (Fig. 3D).
Cortical Sources of the Response to Facelike Patterns.
To estimate the cortical generators of the facelike pattern response identified at the sensor level, we used an anatomical model morphed to newborns’ anatomy (34) to compute a detailed model of the infant head and cortical folds. We then used this forward model to reconstruct a plausible distribution of the cortical origins of our scalp recordings (see Materials and Methods).
The areas associated with the facelike pattern response at the source level (Fig. 3C) comprise a network that appears mostly lateralized to the right hemisphere and includes areas both along the occipitotemporal and the occipitoparietal stream: Along the ventral stream, activity emerges in bilateral occipital regions extending laterally to the right inferior occipital/posterior fusiform gyrus, superiorly toward the right posterior superior temporal sulcus, and anteriorly to the right anterior temporal lobe. A strong activation was also seen in medial posterior regions, including the right precuneus and cuneus. Finally, some activation was observed in the right superior frontal gyrus.
To confirm that the spatial resolution of the source-reconstructed data are sufficiently high to support the anatomical segregation described in Fig. 3C, we tested the spatial spreading of the source reconstruction of surrogate, biologically plausible EEG data generated by simulated oscillatory cortical sources centered in key areas (SI Appendix, Fig. S2A; see SI Appendix, SI Materials and Methods for details). SI Appendix, Fig. S2B shows that the spatial spreading of all the simulated sources was limited to a contiguous neighborhood, supporting the reliability of the anatomical distinction between the activations shown in Fig. 3C.
Response to Scrambled Faces Is Intermediate.
Lastly, we investigated the response to scrambled faces to test the hypothesis that they may yield the same pattern of response to upright faces due to their top-heavy configuration. However, contrary to the comparison of upright vs. inverted faces, the FTR to scrambled faces was not higher as compared with inverted faces (no significant clusters, P > 0.05 for all uncorrected single-channel t tests), nor was it significantly different from upright faces (Pcorr > 0.07). To further explore the nature of this intermediate response, we computed the FTR to scrambled faces in the main cluster associated with the facelike pattern response: Although the average power spectrum is more similar to inverted faces than to upright faces (Fig. 4A), the response to scrambled faces is very variable across subjects (Fig. 4B), being closer to upright faces in some subjects (4/10) and closer to inverted faces in others (6/10).
Discussion
The Mature Cortical Face Network Is Present Early in Newborns.
In this study, we used a frequency-tagging paradigm combined with high-density EEG to show that human newborns display a face-selective neural activation revealed by a higher response to facelike geometric patterns than to tightly controlled visual stimuli.
The estimated cortical sources of such response (Fig. 3C) extend along the occipitotemporal pathway in areas including those found in most adult fMRI studies on face processing; with intracranial EEG recordings using frequency tagging in adults (37); and with fMRI/PET in infants as young as 2 to 6 mo old (23, 24): inferior occipital gyrus (consistent with the location of the occipital face area), fusiform gyrus (even if more posterior than the location of the fusiform face area), posterior superior temporal sulcus, and anterior ventral temporal lobe [see SI Appendix, Fig. S3 for a comparison with the “core system” of face processing in adults (4)]. These results suggest that at least a subset of the cortical face-processing network is already laid down and functional in newborns.
In addition to this occipitotemporal right-lateralized activity, we observe a medial activation centered in the precuneus—a region associated with memory and attention and thus potentially reflecting the higher involvement of these processes in elaborating upright faces vs. inverted faces. Interestingly, face-processing studies in adults using fMRI report a higher activity in the precuneus for familiar vs. unfamiliar faces (38), which may tentatively suggest that the high precuneus activation we observe here could reflect an early-developed familiarity to facelike patterns compared with nonfacelike ones.
Response to Facelike Patterns Does Not Increase with Age.
Although newborns spend most of their time sleeping, when they are awake, the visual stimuli that they are more frequently presented with are upright faces (16). A study by Farroni et al. (39) indicated that the intensity of the near-infrared spectroscopy signal recorded in right occipitotemporal channels while newborns were viewing dynamic faces indeed increased with age in infants from 24 to 120 h after birth. This was taken as evidence that the cortical face-specific response requires frequent exposure to faces to develop. However, the reported correlation is difficult to interpret, as the activation to faces was not directly contrasted with that of a control condition, leaving open the possibility that its increase with age reflected a general maturation of the visual system. Indeed, our results are incompatible with the idea that the face-specific cortical response increases as a function of exposure to faces, because the correlation between age and the facelike pattern response is significantly negative (Fig. 3D). In a speculative attempt to account for this surprising finding, we remark that we used highly simplified facelike geometrical patterns that, for newborns, act as key or supernormal stimuli (in ethological terms; see ref. 40), the sensitivity to which was previously shown to rapidly decrease already within the first month of life (8). One possible explanation is that while such key stimuli are optimally fit for the immature visual system of the newborn in the very first hours of life, experience with real-world complex and variable faces may refine the facelike circuitry such that it rapidly gets more attuned to the real-world features and gradually loses sensitivity to artificial facelike geometrical patterns. This fascinating but speculative possibility deserves further testing with a larger sample of newborns; for example, by comparing the developmental trajectory of the cortical response to facelike patterns and real-world faces.
The Role of Cortical and Subcortical Structures.
An influential theory proposes that newborn preferences for facelike stimuli may be mainly generated by a subcortical route involving the superior colliculus, amygdala, and pulvinar (41). This theory, however, mainly relies on the assumption that the cortical visual route compared with the subcortical one is very immature in newborns and on indirect behavioral evidence that the face-preference phenomenon occurs only through the temporal visual route (42). One alternative possibility is that the processing of visual information proceeds in multiple waves of activation that involve both subcortical and cortical structures (43), a hypothesis supported by the dense connectivity of the structures of the subcortical visual route with multiple areas of the cortex (43) and by recent evidence from resting-state fMRI studies that, by term age, the newborn cortex has reached a highly organized functional architecture (44) and thalamocortical connectivity (45), similar in many aspects to those of the adult.
Our study cannot provide evidence for or against an involvement of the subcortical route in face processing. In fact, because subcortical structures generate extremely weak electrical fields due to their closed-field geometry and because they are far from the scalp, they hardly produce measurable signals at the scalp level (46). Therefore, we believe that an impact of subcortical activity on our EEG results is unlikely and, thus, did not include subcortical areas in our source reconstruction analysis.
However, our source reconstruction results support the hypothesis of a recruitment of a specific set of cortical structures in facelike processing at birth. Since this network overlaps with the adult face-processing circuit, we further speculate that one or more of these cortical areas might be already sensitive enough to facelike stimuli to generate the orientation preference to facelike patterns observed in newborns (7, 8). It is worth noting that this cortical recruitment is fully compatible with an early temporal subcortical route of the visual input (42), alternative to the relatively immature lateral geniculate nucleus/primary visual cortex pathway, because the pulvinar and amygdala are densely connected with (and massively influenced by) multiple cortical areas (43).
Sensitivity of the EEG Frequency-Tagging Paradigm over Behavioral Measures.
Interestingly, while the early behavioral preference for upright facelike patterns compared with inverted ones is systematically observed in newborns by using preferential looking paradigms (whereby two different stimuli are concurrently shown on the screen; e.g., ref. 9), with single central presentations similar to the one used in the current stimulation paradigm, a behavioral preference for faces over nonfacelike controls is typically not detected until 2 mo of age (47). However, EEG responses to our centrally presented single stimuli did indicate a strong FTR difference across conditions, suggesting that, in this case, direct brain measures can be more sensitive compared to behavioral measures.
Faces or Top-Heavy Configurations?
Another result of the current experiment is that of an intermediate response to scrambled faces compared with upright faces and inverted faces. The fact that scrambled faces did not elicit a stronger FTR than inverted faces does not support the hypothesis that face preference reflects a preference for top-heavy configurations (26). In other words, the presence of a top-heavy configuration alone is not sufficient to systematically elicit a facelike neuronal response. However, even if, on average, upright faces elicit a higher FTR compared with scrambled faces, the high intersubject variability suggests that top-heavy stimuli may sometimes be categorized as a face.
The Role of Experience in Early Face Processing.
Recent studies show that after prolonged face deprivation from birth, monkeys do not exhibit any looking preference for faces (15, 48) and, unlike normally reared monkeys, they do not develop any fMRI-measured face-selective domains (48). Our findings are not in contradiction with these results for two main reasons. First, both Arcaro et al.’s (48) and Sugita’s (15) studies tested face selectivity in monkeys reared in face deprivation (interacting only with masked human caregivers) for at least the first 3 mo after birth. We believe that face selectivity at the test time is likely to be strongly influenced by the experience of face deprivation during this long and critical period of development, whereas it is poorly informative about face selectivity at birth. Interestingly, both the fMRI responses and looking times in ref. 48 indicate that during the face-deprivation period, monkeys developed a high selectivity for hands and body parts, the most relevant stimuli in the interaction with their caregivers. One potential speculation is that this environment-specific social learning might have caused the “unlearning” of their early predisposition to encode faces (if there was such a predisposition), a form of high functional plasticity that systematically occurs in animals with sensory deprivation (49); notably, face selectivity is not irreversibly lost but is recovered upon late exposure to faces, even after 2 y of face deprivation (15). On the other hand, contrary to face-deprived monkeys, our newborn subjects did have a normal, albeit very limited, exposure to human faces, and this might have been sufficient to boost potentially predetermined face-specific cortical responses.
Second, fMRI studies in normally reared monkeys failed to reveal significant “face patches” before 6 mo of age (50). However, because monkeys show a clear face-biased preference much earlier on, the presence of a cortical patch that is entirely and selectively dedicated to faces is not necessary for encoding (and preferring) faces. What, then, is the neural underpinning of face preference in monkeys? There are two possibilities that are coherent with the absence of face patches: Face-responsive neurons might already exist at birth but (i) they might be less spatially segregated from the nonface-responding neurons compared with the adult’s cortical organization, or (ii) their degree of face selectivity might be weaker than that observed in adults’ face neurons [as is observed in 4- to 6-mo-old human infants (23)]. Both scenarios are likely to provide null results with the mass-univariate subtraction-based fMRI data analyses performed in refs. 48 and 50, whereas face-specific responses might be revealed using multivariate pattern analysis methods, which, unfortunately, were not performed in the aforementioned studies. Our study, which does not rely on the assumption of the amount of spatial segregation of face-selective neurons and which integrates a very powerful low-level visual control (inverted faces), clearly shows that it is possible to detect face-specific neural responses as early as 1 to 3 d from birth.
Future Directions.
Newborns spend most of their time sleeping, and during the rare periods in which they are calm and awake, their visual attention typically lasts no more than 3 to 5 min (27). Here, we show that the frequency-tagging paradigm provides a valid tool for measuring in newborns high SNR brain responses to multiple stimulus-specific conditions with very short stimulus presentation (around 40 s per condition), confirming results obtained with older infants (21, 51) and opening the way to investigate the neural substrates of other core perceptual/cognitive functions in this very special population. The high statistical significance of the facelike bias effect, reflected in its presence for each single subject, suggests that our experimental protocol, even in its shorter version limited to the presentation of upright and inverted faces, might be used as a biomarker to test the sensitivity to facelike patterns in populations at risk (like autism spectrum disorder) as a complement to behavioral tests on visual social predispositions (52).
Materials and Methods
The study was approved by the local ethical committee for clinical research (Comitato Etico per le Sperimentazioni Cliniche, Azienda Provinciale Servizi Sanitari, Province of Trento, Italy) and was performed in the maternity ward of Rovereto Hospital Santa Maria del Carmine. Parents were informed about the content and goal of the study and gave their written informed consent. The data that support the findings of this study are available from the corresponding authors upon reasonable request by individual scholars.
Subjects.
Ten newborns (six males; mean age 60 ± 22 h, range 15 to 96 h) were included. All were healthy [APGAR(1 min) ≥ 8, APGAR(5 min) = 10 for all subjects], born full term (gestation age, 39.7 ± 1.5 wk), and of normal birthweight (average weight, 3.41 ± 0.28 kg). Forty-four additional newborns participated but were excluded either because they did not complete the study (criteria: attend all three stimulus conditions for at least 20 s each) due to inattentiveness (18), falling asleep (16), or crying (5) or because their data contained too many EEG artifacts (mainly due to movements or high electrode impedance) (5).
Stimuli.
See SI Appendix, SI Materials and Methods for details. In brief, visual stimuli (Fig. 1, Top) consisted of a white head-shaped form containing three black squares, and differed only in the spatial configuration of the three squares to form the three stimuli (upright face, inverted face, and scrambled face). Stimuli were presented dynamically with sinusoidal contrast modulation (0 to 100%) at a rate of 0.8 Hz, overlapped onto a weakly contrasted dynamic background (Fig. 1, Bottom) consisting of a flickering white-noise image. We used sinusoidal contrast modulation instead of squared on–off dynamics, both to minimize nonlinear effects in the brain frequency response (28) and to make the stimulation more pleasant to the babies (21). The slow presentation rate (0.8 Hz) was chosen to ensure that newborns fully perceived the stimulus at each cycle of the periodic, peekaboo-like presentation.
Experimental Protocol.
Newborns were tested in a calm, dimly illuminated space in the maternity ward, seated on the lap of a trained researcher in front of an LCD screen (60 × 33.8 cm; distance from eyes to screen: about 30 cm) while wearing an EEG cap (see SI Appendix, SI Materials and Methods for details about the EEG system). Video recording from a hidden camera on the top of the screen ensured on-line monitoring of the infant. The newborn’s parents, when present, were off the sight of the infant (separated by a curtain), and instructed to keep silent during the recordings.
Each trial started with a distracter (a gray spiral looming toward the center of the screen on a reddish background). As soon as the newborn started to fixate the center of the screen, stimulation started with 1 s of flickering background, followed by the periodic presentation of one of the three stimuli. Each condition was presented for 40 cycles (50 s) or until the subject stopped fixating and became bored or fussy. The trial ended with 1 s of flickering background followed by a blank screen. For each subject, the three conditions were presented in random order (counterbalanced across subjects). If the newborn kept her/his attention after each triplet of conditions, the same triplet was presented again, up to three times.
Fixation intervals were recomputed off-line by an experienced researcher (E.D.G.) who reviewed the video recordings blindly with respect to the experimental conditions. Newborns had an average fixation time of 43.4 s per condition. There was no statistical difference among fixation intervals in the three conditions [F(2,18) = 0.24, P = 0.74].
EEG Data Analysis.
Data analysis was performed with EEGLAB (53), FieldTrip (54), Brainstorm (55), and custom-made software based on MATLAB R2016b. EEG data were preprocessed for artifacts and segmented in blocks corresponding to fixation intervals (see SI Appendix, SI Materials and Methods for details).
Frequency-Tagging Analysis.
To obtain a high-frequency resolution of the power spectrum with one bin centered on the stimulation frequency (0.8 Hz), epoch length was set to exactly eight stimulation cycles (10 s), resulting in a frequency resolution of 0.1 Hz. EEG data from each block were segmented in partially overlapping epochs of 10 s (overlap varied between one-half and three-fourths of epoch length to include all time points). For each electrode, the Fourier transform of each epoch was calculated using a fast Fourier transform algorithm (MATLAB function FFT). The power spectrum was calculated from these Fourier coefficients as the average over epochs of the single-epoch power spectrum: . The FTR at the tag frequency (0.8 Hz) was calculated as the ratio between the power spectrum at the tagged frequency and the value at 0.8 Hz of the power-law fit of the power spectrum estimated from the six neighboring frequency bins (±0.3 Hz), where the power-law fit was computed by fitting a line to the logarithm of the power at the six neighboring frequency bins (MATLAB function Polyfit). It is worth noting that, due to the steep -like power law of the power spectrum in newborns in the low-frequency interval analyzed here (0.5 to 1.1 Hz) (35), the popular method to estimate the background power spectrum at the tag frequency by simply averaging over neighboring frequency bins (56) overestimates the background power (and therefore underestimates the FTR) because the power spectrum is much steeper for lower-frequency compared with higher-frequency bins around the tag frequency.
Statistical Analysis.
The frequency-tagging effect was evaluated by comparing the logarithm of the power at the tag frequency with the logarithm of the background power estimated by the power-law fit described above. Differences between conditions were evaluated by comparing the logarithm of the relative FTRs. We tested the statistical significance of these effects with the nonparametric cluster-based test (36) implemented in FieldTrip (54). This method allows statistical testing with no need of a priori selection of regions of interest because it controls for multiple comparisons by clustering neighboring channel pairs that exhibit statistically significant effects (test used at each channel point, dependent-samples t statistics; threshold, P = 0.05; one-sided for the frequency-tagging effect, two-sided for the differences between conditions) and using a permutation test to evaluate the statistical significance at the cluster level (Monte Carlo method, 2,000 permutations for each test). The P value of each statistically significant cluster is indicated as Pcorr to mark that it is corrected for multiple comparisons. The effect size is estimated with a post hoc analysis on the statistically significant cluster by computing Cohen’s d as the ratio between the mean and the SD (across subjects) of the effect averaged over all of the electrodes of the cluster.
Source Reconstruction.
See SI Appendix, SI Materials and Methods for details about the source reconstruction model. Source-level FTRs were estimated as follows: (i) For each subject, source-level time series were reconstructed from the segmented EEG data on the 8,014 sources obtained from the wMNE reconstruction in Brainstorm; (ii) log(FTR) was estimated at the source level by using the same frequency-tagging analysis used at the sensor level; and (iii) the resulting source signal was spatially smoothed (10 mm).
For each contrast of interest, a paired t test was run at each source location, and the corresponding significant clusters (P < 0.05 uncorrected) are reported on a template cortex smoothed at 30%. Importantly, the t test at the source level is only used to properly describe the source distribution of the statistically significant effect established at the sensor level, not for a second statistical test at the source level; therefore, no correction for multiple comparison is required (57).
Supplementary Material
Acknowledgments
We thank the staff of the Maternity Unit of Rovereto Hospital Santa Maria del Carmine and the parents of the newborns involved in the study for their collaboration; Mariapia Giuseppa Piccininni and Roberta Eccher for help in data collection; and Sampsa Vanhatalo and Anton Tokariev for sharing their realistic newborn head model. This work was performed within a collaboration agreement between the Center for Mind/Brain Sciences (University of Trento, Italy) and the Azienda Provinciale Servizi Sanitari (Province of Trento, Italy) (Protocol 0124397, September 18, 2015). This work was supported by grants from the European Research Council (ERC) under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC Advanced Grant PREMESOR (295517) (to G.V.); the Fondazione Caritro Grant Biomarker Disordini dello Spettro Autistico (G.V.); and the Italian Programmi di Rilevante Interesse Nazionale (PRIN 2015) “Neural bases of animacy detection, and their relevance to the typical and atypical development of the brain”) (to G.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812419116/-/DCSupplemental.
References
- 1.Rhodes G, Calder A, Johnson M, Haxby JV, editors. Oxford Handbook of Face Perception. Oxford Univ Press; Oxford: 2011. [Google Scholar]
- 2.Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 4.Haxby JV, Gobbini MI. Distributed neural systems for face perception. In: Rhodes G, Calder A, Johnson M, Haxby JV, editors. Oxford Handbook of Face Perception. Oxford Univ Press; Oxford: 2011. pp. 93–109. [Google Scholar]
- 5.Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc Natl Acad Sci USA. 2008;105:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung C-C, et al. Functional mapping of face-selective regions in the extrastriate visual cortex of the marmoset. J Neurosci. 2015;35:1160–1172. doi: 10.1523/JNEUROSCI.2659-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goren CC, Sarty M, Wu PYK. Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics. 1975;56:544–549. [PubMed] [Google Scholar]
- 8.Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- 9.Valenza E, Simion F, Cassia VM, Umiltà C. Face preference at birth. J Exp Psychol Hum Percept Perform. 1996;22:892–903. doi: 10.1037//0096-1523.22.4.892. [DOI] [PubMed] [Google Scholar]
- 10.Cassia VM, Turati C, Simion F. Can a nonspecific bias toward top-heavy patterns explain newborns’ face preference? Psychol Sci. 2004;15:379–383. doi: 10.1111/j.0956-7976.2004.00688.x. [DOI] [PubMed] [Google Scholar]
- 11.Farroni T, et al. Newborns’ preference for face-relevant stimuli: Effects of contrast polarity. Proc Natl Acad Sci USA. 2005;102:17245–17250. doi: 10.1073/pnas.0502205102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid VM, et al. The human fetus preferentially engages with face-like visual stimuli. Curr Biol. 2017;27:1825–1828.e3. doi: 10.1016/j.cub.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 13.Di Giorgio E, et al. Filial responses as predisposed and learned preferences: Early attachment in chicks and babies. Behav Brain Res. 2017;325:90–104. doi: 10.1016/j.bbr.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Rosa Salva O, Farroni T, Regolin L, Vallortigara G, Johnson MH. The evolution of social orienting: Evidence from chicks (Gallus gallus) and human newborns. PLoS One. 2011;6:e18802. doi: 10.1371/journal.pone.0018802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugita Y. Face perception in monkeys reared with no exposure to faces. Proc Natl Acad Sci USA. 2008;105:394–398. doi: 10.1073/pnas.0706079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fausey CM, Jayaraman S, Smith LB. From faces to hands: Changing visual input in the first two years. Cognition. 2016;152:101–107. doi: 10.1016/j.cognition.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halit H, de Haan M, Johnson MH. Cortical specialisation for face processing: Face-sensitive event-related potential components in 3- and 12-month-old infants. Neuroimage. 2003;19:1180–1193. doi: 10.1016/s1053-8119(03)00076-4. [DOI] [PubMed] [Google Scholar]
- 18.Peykarjou S, Hoehl S. Three-month-olds’ brain responses to upright and inverted faces and cars. Dev Neuropsychol. 2013;38:272–280. doi: 10.1080/87565641.2013.786719. [DOI] [PubMed] [Google Scholar]
- 19.Halit H, Csibra G, Volein A, Johnson MH. Face-sensitive cortical processing in early infancy. J Child Psychol Psychiatry. 2004;45:1228–1234. doi: 10.1111/j.1469-7610.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- 20.Adibpour P, Dubois J, Dehaene-Lambertz G. Right but not left hemispheric discrimination of faces in infancy. Nat Hum Behav. 2018;2:67–79. doi: 10.1038/s41562-017-0249-4. [DOI] [PubMed] [Google Scholar]
- 21.de Heering A, Rossion B. Rapid categorization of natural face images in the infant right hemisphere. eLife. 2015;4:e06564. doi: 10.7554/eLife.06564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossion B, Jacques C. The N170: Understanding the time course of face perception in the human brain. In: Kappenman ES, Luck SJ, editors. The Oxford Handbook of Event-Related Potential Components. Oxford Univ Press; Oxford: 2011. pp. 115–142. [Google Scholar]
- 23.Deen B, et al. Organization of high-level visual cortex in human infants. Nat Commun. 2017;8:13995. doi: 10.1038/ncomms13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzourio-Mazoyer N, et al. Neural correlates of woman face processing by 2-month-old infants. Neuroimage. 2002;15:454–461. doi: 10.1006/nimg.2001.0979. [DOI] [PubMed] [Google Scholar]
- 25.Braddick O, Atkinson J. Development of human visual function. Vision Res. 2011;51:1588–1609. doi: 10.1016/j.visres.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Turati C, Simion F, Milani I, Umiltà C. Newborns’ preference for faces: What is crucial? Dev Psychol. 2002;38:875–882. [PubMed] [Google Scholar]
- 27.Prechtl HFR, O’Brien MJ. Behavioural states of the full-term newborn. The emergence of a concept. In: Stratton P, editor. Psychobiology of the Human Newborn. Wiley; Chichester, UK: 1982. pp. 53–73. [Google Scholar]
- 28.Norcia AM, Appelbaum LG, Ales JM, Cottereau BR, Rossion B. The steady-state visual evoked potential in vision research: A review. J Vis. 2015;15:4. doi: 10.1167/15.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atkinson J, Braddick O, French J. Contrast sensitivity of the human neonate measured by the visual evoked potential. Invest Ophthalmol Vis Sci. 1979;18:210–213. [PubMed] [Google Scholar]
- 30.Braddick OJ, Wattam-Bell J, Atkinson J. Orientation-specific cortical responses develop in early infancy. Nature. 1986;320:617–619. doi: 10.1038/320617a0. [DOI] [PubMed] [Google Scholar]
- 31.Odabaee M, et al. Neonatal EEG at scalp is focal and implies high skull conductivity in realistic neonatal head models. Neuroimage. 2014;96:73–80. doi: 10.1016/j.neuroimage.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Despotovic I, et al. Relationship of EEG sources of neonatal seizures to acute perinatal brain lesions seen on MRI: A pilot study. Hum Brain Mapp. 2013;34:2402–2417. doi: 10.1002/hbm.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tokariev A, Vanhatalo S, Palva JM. Analysis of infant cortical synchrony is constrained by the number of recording electrodes and the recording montage. Clin Neurophysiol. 2016;127:310–323. doi: 10.1016/j.clinph.2015.04.291. [DOI] [PubMed] [Google Scholar]
- 34.Tokariev A, et al. Preterm birth changes networks of newborn cortical activity. Cereb Cortex. 2019;29:814–826. doi: 10.1093/cercor/bhy012. [DOI] [PubMed] [Google Scholar]
- 35.Fransson P, et al. Early development of spatial patterns of power-law frequency scaling in FMRI resting-state and EEG data in the newborn brain. Cereb Cortex. 2013;23:638–646. doi: 10.1093/cercor/bhs047. [DOI] [PubMed] [Google Scholar]
- 36.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 37.Jonas J, et al. A face-selective ventral occipito-temporal map of the human brain with intracerebral potentials. Proc Natl Acad Sci USA. 2016;113:E4088–E4097. doi: 10.1073/pnas.1522033113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45:32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Farroni T, et al. Infant cortex responds to other humans from shortly after birth. Sci Rep. 2013;3:2851. doi: 10.1038/srep02851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vallortigara G. Core knowledge of object, number, and geometry: A comparative and neural approach. Cogn Neuropsychol. 2012;29:213–236. doi: 10.1080/02643294.2012.654772. [DOI] [PubMed] [Google Scholar]
- 41.Johnson MH. Subcortical face processing. Nat Rev Neurosci. 2005;6:766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- 42.Simion F, Valenza E, Umiltà C, Dalla Barba B. Preferential orienting to faces in newborns: A temporal-nasal asymmetry. J Exp Psychol Hum Percept Perform. 1998;24:1399–1405. doi: 10.1037//0096-1523.24.5.1399. [DOI] [PubMed] [Google Scholar]
- 43.Pessoa L, Adolphs R. Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doria V, et al. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci USA. 2010;107:20015–20020. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toulmin H, et al. Specialization and integration of functional thalamocortical connectivity in the human infant. Proc Natl Acad Sci USA. 2015;112:6485–6490. doi: 10.1073/pnas.1422638112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wadman WJ, Da Silva FL. Biophysical aspects of EEG and MEG generation. In: Schomer DL, Da Silva FL, editors. Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. 7th Ed. Oxford Univ Press; Oxford: 2017. pp. 89–103. [Google Scholar]
- 47.Maurer D, Barrera M. Infants’ perception of natural and distorted arrangements of a schematic face. Child Dev. 1981;52:196–202. [PubMed] [Google Scholar]
- 48.Arcaro MJ, Schade PF, Vincent JL, Ponce CR, Livingstone MS. Seeing faces is necessary for face-domain formation. Nat Neurosci. 2017;20:1404–1412. doi: 10.1038/nn.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blakemore C, Cooper GF. Development of the brain depends on the visual environment. Nature. 1970;228:477–478. doi: 10.1038/228477a0. [DOI] [PubMed] [Google Scholar]
- 50.Livingstone MS, et al. Development of the macaque face-patch system. Nat Commun. 2017;8:14897. doi: 10.1038/ncomms14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kabdebon C, Pena M, Buiatti M, Dehaene-Lambertz G. Electrophysiological evidence of statistical learning of long-distance dependencies in 8-month-old preterm and full-term infants. Brain Lang. 2015;148:25–36. doi: 10.1016/j.bandl.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Di Giorgio E, et al. NIDA-Network Difference in visual social predispositions between newborns at low- and high-risk for autism. Sci Rep. 2016;6:26395. doi: 10.1038/srep26395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput Intell Neurosci. 2011;2011:879716. doi: 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buiatti M, Peña M, Dehaene-Lambertz G. Investigating the neural correlates of continuous speech computation with frequency-tagged neuroelectric responses. Neuroimage. 2009;44:509–519. doi: 10.1016/j.neuroimage.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 57.Gross J, et al. Good practice for conducting and reporting MEG research. Neuroimage. 2013;65:349–363. doi: 10.1016/j.neuroimage.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.