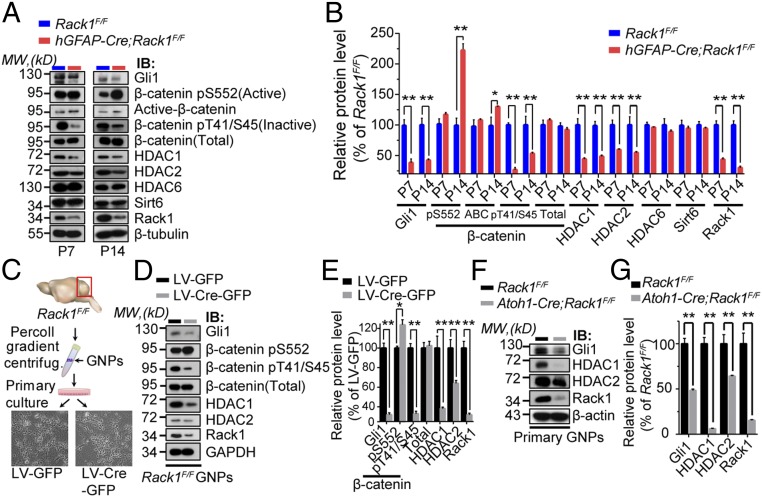
Fig. 5.
Conditional ablation of Rack1 causes simultaneously overactivated Wnt/β-catenin signaling but impaired Shh signaling pathways. (A) Representative Western blots examining downstream Wnt/β-catenin and Shh signaling protein expression in control and hGFAP-Cre;Rack1F/F mutant cerebella at P7 and P14, respectively. IB, immunoblot; MW, molecular weight. (B) Quantitative analysis indicates elevated levels of the active form but decreased levels of the inactive form of β-catenin and reduced Gli1 and HDAC1/HDAC2 expression in hGFAP-Cre;Rack1F/F mutants. HDAC6 and Sirt6 expression was not significantly different between controls and mutants. Significantly reduced expression of Rack1 was detected in mutant (mean ± SEM; **P < 0.001, *P < 0.01, n = 3). (C) Schematic showing the primary culture of GNPs from Rack1F/F mice and infection with LV-Cre-GFP or LV-GFP control LV. (D and E) Quantitative Western blot analysis indicates the significantly increased active form (pS552), but decreased inactive form (pT41/S45), of β-catenin in primary Rack1F/F GNPs after LV-Cre-GFP infection compared with LV-GFP–infected control cells (mean ± SEM; **P = 0.0226 and **P = 0.0001, respectively; n = 3). The total level of β-catenin was unaltered (P = 0.6028, n = 3). The expression of Gli1 and HDAC1/HDAC2 was also significantly reduced after LV-Cre-GFP infection (mean ± SEM; **P = 0.0001, **P = 0.0009, and **P = 0.0025, respectively; n = 3). Rack1 was used as a positive control (**P = 0.0007, n = 3). (F and G) Representative Western blots and quantification showing reduced expression of downstream Shh signaling molecules in the Atoh1-Cre;Rack1F/F mutant cerebellum at P7 (mean ± SEM; **P < 0.0001,**P = 0.0039, and **P = 0.0002, respectively; n = 3).