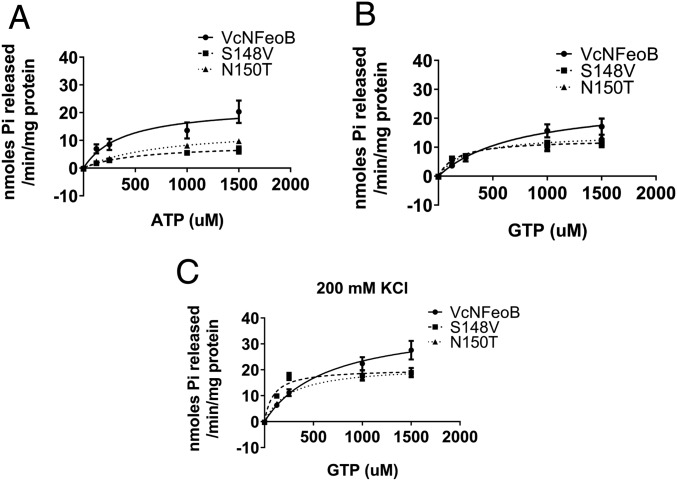
Fig. 6.
Ser148 and Asn150 of VcNFeoB play a role in ATPase, but not GTPase, activity. (A) Mutants of the G5 motif (S148V and N150T) showed decreased ATPase activity compared with wild-type VcNFeoB in the absence of potassium. Both in the absence (B) and presence (C) of potassium, the maximum GTPase activity of the G5 motif mutants was slightly lower compared with wild type, but their GTP-binding affinity was higher, making them more similar to EcNFeoB (Fig. 1C).