Table 1.
Optimization of Reaction Conditionsa
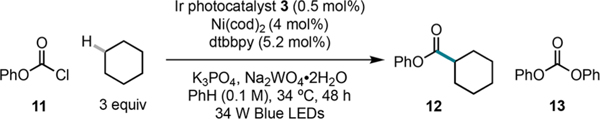 | |||
|---|---|---|---|
| Entry | Deviation from standard condition | Yield 12 (%)b | Yield 13 (%)c |
| 1 | None | 66 | 28 |
| 2 | 1 equiv of cyclohexane | 44 | 33 |
| 3 | no photocatalyst | 0 | 48 |
| 4 | no ligand | 3 | 27 |
| 5 | no nickel | 0 | 17 |
| 6 | no light | 0 | 34 |
| 7 | 26 °C | 46 | 17 |
| 8 | 40 °C | 55 | 32 |
| 9 | no Na2WO4·2H2Od | 39 | 19 |
| 10 | no Na2WO4·2H2Od, 72 h | 62 | 22 |
| 11 | Na2SiO3·5H2O instead of Na2WO4·2H2O | 57 | 25 |
| 12 | no K3PO4d | 58 | 35 |
| 13 | no K3PO4, no Na2WO4·2H2O | 17 | 11 |
K3PO4 (2 equiv); Na2WO4·2H2O (1 equiv).
Yields determined by 1H NMR using 4-fluoroanisole as an external standard.
Yield determined by GC analysis based on consumption of 2 equiv of chloroformate for production of 1 equiv of DPC.
3 equiv of base used.
