Abstract
The layer exchange technique enables high-quality multilayer graphene (MLG) on arbitrary substrates, which is a key to combining advanced electronic devices with carbon materials. We synthesize uniform MLG layers of various thicknesses, t, ranging from 5 nm to 200 nm using Ni-induced layer exchange at 800 °C. Raman and transmission electron microscopy studies show the crystal quality of MLG is relatively low for t ≤ 20 nm and dramatically improves for t ≥ 50 nm when we prepare a diffusion controlling Al2O3 interlayer between the C and Ni layers. Hall effect measurements reveal the carrier mobility for t = 50 nm is 550 cm2/Vs, which is the highest Hall mobility in MLG directly formed on an insulator. The electrical conductivity (2700 S/cm) also exceeds a highly oriented pyrolytic graphite synthesized at 3000 °C or higher. Synthesis technology of MLG with a wide range of thicknesses will enable exploration of extensive device applications of carbon materials.
Introduction
Multilayer graphene (MLG) has excellent characteristics, such as high electrical/thermal conductivities and current-carrying capacity exceeding that of Cu1–4. Therefore, application of MLG on arbitrary substrates is expected in various applications, including transparent electrodes, low-resistance wiring, and heat spreaders. As the required MLG thickness depends on the application, a technique for controlling the thickness of high-quality MLG film is essential.
High-quality graphene and MLG have been produced on arbitrary substrates using transfer techniques5 and chemical vapor deposition6–11. Some of these techniques can precisely control the numbers of graphene layers; however, there is difficulty forming thick MLG in the tens of nanometers. Against this backdrop, metal-induced solid-phase crystallization of amorphous carbon (a-C) or polymers has attracted increased attention owing to the direct synthesis of MLG on insulators12–26. Some of these techniques have allowed synthesis of thick (>5 nm) MLG by controlling the initial thickness of a-C17–26. However, a problem has existed with the uniformity of the MLG layer, which makes it difficult to systematically evaluate the electrical properties of such layers.
Metal-induced layer exchange (MILE) has been actively studied in the field of group-IV semiconductors, including Si27–31, Ge32–37, and SiGe38,39. In MILE, an amorphous semiconductor layer crystallizes through “layer exchange” between the amorphous layer and a catalyst metal layer. The thickness design of the initial metal layer can easily control the resulting semiconductor layer27,31,35. We found the layer exchange occurs in the Co-C and Ni-C systems and fabricated a uniform 50-nm-thick MLG on an insulator40,41. Furthermore, we employed a diffusion controlling interlayer (IL) between C and Ni, which suppresses the nucleation of the MLG and results in enlargement of MLG grains42. In MILE, the initial metal thickness strongly influences the crystallinity of the resulting semiconductor layer31,38. This study first clarifies the effect of film thickness on the crystallinity and electrical properties of MLG on an insulator. The result shows a carrier mobility of 550 cm2/Vs and an electrical conductivity of 2700 S/cm; the highest values among most MLG layers directly formed on insulators.
Results
As shown in Fig. 1a, all samples started with a stacked structure of an a-C/Ni/SiO2 substrate and were then annealed at 800 °C for 1 h in ambient Ar. We prepared the samples with and without a diffusion controlling Al2O3 IL, which improves the crystal quality of MLG42. The thickness of the initial Ni layer determines the resulting MLG thickness, t, after layer exchange41,42. We varied t from 5 nm to 200 nm to investigate the effects of t on the crystal and electrical properties of the MLG. For all samples, MLG layers were obtained on the substrate via layer exchange. As representatively shown in Fig. 1b, thin MLG layers (t ≤ 10 nm) exhibited transparency. The scanning electron microscopy (SEM) image in Fig. 1c shows that although the MLG layer has submicron-size voids, it covers nearly the entire substrate. We note the Ni concentration in the MLG layer is below the detection limit of EDX (~1%).
Figure 1.
Typical example of layer exchange. (a) Schematic of the sample preparation procedure. (b) Photograph and (c) SEM image of the sample for t = 5 nm without IL after Ni removal.
Figure 2a,b show that Raman spectra of the back side of the samples have peaks at approximately 1350, 1580, and 2700 cm−1, corresponding to the D (disordered mode), G (graphitic mode), and 2D (D mode overtone) peaks in the graphitic structure, respectively. This means layer exchange between the C and Ni layers occurred and MLG formed on the SiO2 glass substrate in all samples. In the Raman spectra, the intensity ratio of the G to D peaks (IG/ID) in the spectra corresponds to the crystal quality of MLG43. Figure 2c indicates the IG/ID ratio strongly depends on t, and with or without the IL. For the samples without the IL, the IG/ID ratio shows the highest value of 7 at t = 100 nm. For the samples with the IL, the IG/ID ratio is as low as for the samples without the IL for t ≤ 20 nm, while it dramatically increases for t ≥ 50 nm and exceeds 20. The crystal quality of the MLG, estimated from the IG/ID ratio, likely reflects the contamination from the substrate and the grain size enlargement by inserting the IL42. Although the IG/ID ratio of 20 is still lower than that of MLG formed by the transfer technique or high-temperature CVD4, this value is the highest among MLG synthesized on insulators by metal-induced solid-phase crystallization18,19,23,25.
Figure 2.
Raman study of MLG formed by layer exchange. Raman spectra obtained from back side of the samples before Ni removal (a) without and (b) with the IL. (c) G/D intensity ratio of the samples determined by the Raman spectra shown in (a,b), as a function of t.
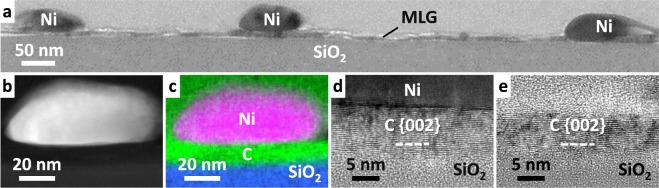
The detailed cross-sectional structure of the sample for t = 10 nm, which is a typical thin transparent film, was investigated using an analytical transmission electron microscope (TEM). Figure 3a shows the MLG formed on the entire substrate. Figure 3b,c show Ni on the MLG layer is particle-shaped, whereas the sample with t = 50 nm has a continuous Ni layer41,42. Considering that the MLG layer is uniformly formed, the Ni particles are likely attributed to the agglomeration of a thin Ni layer moved on MLG after layer exchange. Figure 3d,e show {002} oriented MLG forms on the SiO2 substrate in both the Ni-contacted and non-Ni-contacted regions. The MLG in the non-Ni-contacted region is slightly thinner than that in the Ni-contacted region, probably because of the C evaporation during annealing. Figure 3d,e indicate the crystal structure of the thin MLG is disordered compared with the sample with an IL42. We note that, for t = 50 nm, the grain size of the MLG layer without an IL was a few hundred nm and that with an IL was a few μm41,42. These results account well for the Raman study (Fig. 2c). For high-quality MLG, such as that synthesized by thermal decomposition of a SiC substrate, TEM observation can identify the stacking structure (e.g. AA and AB stacking)44,45. Unfortunately, because the current MLG is small-grained polycrystalline where the grains are randomly oriented in the in-plane direction42, it is difficult to identify the stacking.
Figure 3.
Characterization of the cross-section of the sample for t = 10 nm without the IL before Ni removal. (a) Bright-field TEM image. (b) HAADF-STEM image and (c) EDX elemental map of an Ni-contacted region. High-resolution lattice images of the MLG layer showing (d) an Ni-contacted region and (e) a non-Ni-contacted region.
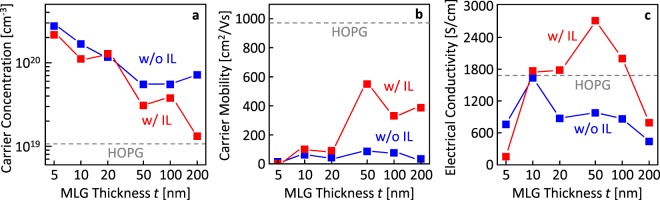
Figure 4 shows the electrical properties of the MLG strongly depend on t, and with or without the IL. Figure 4a shows the carrier concentration of the MLG decreases with the increase of t and approaches the value of a highly oriented pyrolytic graphite (HOPG) with a low mosaic degree of 0.4°, especially for the sample with the IL. Figure 4b shows the carrier mobility dramatically increases for the t ≥ 50 nm samples with the IL, whereas it remains low in the whole range of t for the samples without the IL. The carrier mobility exhibits the maximum value of 550 cm2/Vs for the t = 50 nm sample with the IL, which is the highest Hall mobility among MLGs directly formed on an insulator. Figure 4c shows the behavior of the electrical conductivity reflects that of the carrier concentration and carrier mobility. The electrical conductivity exhibits the maximum value of 2700 S/cm for the t = 50 nm sample with the IL, which exceeds that of the HOPG synthesized at 3000 °C or higher.
Figure 4.
Electrical properties of the MLG layers after Ni removal as a function of t. (a) Carrier concentration. (b) Carrier mobility. (c) Electrical conductivity. The data of a HOPG are shown as dotted lines.
Discussion
We have controlled the thickness of MLG on an insulator formed by layer exchange with and without the IL, resulting in a high electrical conductivity. Considering the fact that the behavior of the carrier mobility (Fig. 4b) is the same as that of the IG/ID ratio in the Raman spectra (Fig. 2c), the carrier mobility clearly reflects the crystal quality of the MLG. The carrier mobility, that is, the crystal quality of the MLG is much higher than any other MLG directly formed on an insulator; however, does not reach the HOPG. The electrical conductivity of the MLG exceeds that of HOPG with the aid of autodoping. Although the reasons for the excessive carriers for the MLG are still unclear, when we assume defects in MLG produce carriers, the carrier concentration behavior is almost consistent with the Raman study.
In conclusion, the crystal quality and electrical properties of the MLG, formed on an insulator by layer exchange at 800 °C, strongly depended on t, especially when preparing the IL. The crystal quality was relatively low for t ≤ 20 nm; however, it dramatically improved for t ≥ 50 nm with the IL. As a result, the sample for t = 50 nm with the IL exhibited the maximum carrier mobility (550 cm2/Vs), and the highest Hall mobility among MLG directly formed on an insulator. The electrical conductivity (2700 S/cm) even exceeded the high-quality HOPG (mosaic degree: 0.4°). Thus, the layer exchange method allows us to form MLG with a wide range of thicknesses on arbitrary substrates. This opens the door for a broad range of applications that combine advanced electronic devices with carbon materials.
Methods
Sample preparation
Ni thin films with thicknesses of 5, 10, 20, 50, 100, and 200 nm were prepared on SiO2 glass substrates. Subsequently, Al2O3 (thickness: 2 nm) was employed as the diffusion control IL42. Then, a-C thin films were prepared, wherein the thickness ratio of C:Ni = 3:2 (e.g., C:Ni = 300:200 nm). All depositions were carried out using radio-frequency (RF) magnetron sputtering (base pressure: 3.0 × 10−4 Pa) with an Ar plasma. During the deposition, the substrate temperature was room temperature for Al2O3 and 200 °C for Ni and C. The RF power was set to 100 W for C and 50 W for Ni and Al2O3. For comparison, we prepared the samples without the Al2O3 IL. Samples were annealed at 800 °C for 1 h in an Ar ambient to induce layer exchange growth. The sample was dipped in a diluted HNO3 solution (10% HNO3) for 30 min to remove Ni moved to the top layer.
Material characterization
SEM analyses were performed using a JEOL JSM-7001F with an EDX spectrometer (JEOL JEO-2300). Raman spectroscopy was performed using a JASCO NRS-5100, wherein the laser wavelength was 532 nm and the spot size was 5 µm. TEM analyses were performed using an analytical TEM, FEI Tecnai Osiris, operating at 200 kV, equipped with an EDX spectrometer (FEI Super-X system). The cross-sectional TEM sample was prepared using the conventional focused ion beam method. Hall effect measurement was performed with the Van der Pauw method using the Bio-Rad HL5500PC system. Carrier mobility, carrier concentration, and electrical conductivity were averaged over five measurements for each sample.
Acknowledgements
This work was financially supported by JSPS KAKENHI (No. 18K18844), JSPS Research Fellow (No. 18J20904), Advanced Technology Institute Research Grants (RG2906), and JST PRESTO (No. JPMJPR17R7). The authors are grateful to Dr. T. Sakurai at the University of Tsukuba for assistance with the Hall effect measurements. Some experiments were conducted at the International Center for Young Scientists at NIMS and the Nanotechnology Platform at the University of Tsukuba and AIST.
Author Contributions
K.T. conceived and designed the experiments. H.M. and Y.N. conducted the sample fabrication and the majority of the analyses. N.S. and N.Y conducted the TEM analyses. K.T. and T.S. managed and supervised the research project. All authors discussed the results and commented on the manuscript.
Data Availability
The data used in this study are available upon reasonable request from the corresponding author K.T. (toko@bk.tsukuba.ac.jp).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Biswas S, Drzal LT. Multilayered nano-architecture of variable sized graphene nanosheets for enhanced supercapacitor electrode performance. ACS Appl. Mater. Interfaces. 2010;2:2293–2300. doi: 10.1021/am100343a. [DOI] [PubMed] [Google Scholar]
- 2.Balandin AA. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011;10:569–581. doi: 10.1038/nmat3064. [DOI] [PubMed] [Google Scholar]
- 3.Murali R, Yang Y, Brenner K, Beck T, Meindl JD. Breakdown current density of graphene nanoribbons. Appl. Phys. Lett. 2009;94:243114. doi: 10.1063/1.3147183. [DOI] [Google Scholar]
- 4.Kim KS, et al. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature. 2009;457:706–710. doi: 10.1038/nature07719. [DOI] [PubMed] [Google Scholar]
- 5.Novoselov KS, et al. Electric field effect in atomically thin carbon films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 6.Su C-Y, et al. Direct formation of wafer scale graphene thin layers on insulating substrates by chemical vapor deposition. Nano Lett. 2011;11:3612–3616. doi: 10.1021/nl201362n. [DOI] [PubMed] [Google Scholar]
- 7.Kato T, Hatakeyama R. Direct growth of doping-density-controlled hexagonal graphene on SiO2 substrate by rapid-heating plasma CVD. ACS Nano. 2012;6:8508–8515. doi: 10.1021/nn302290z. [DOI] [PubMed] [Google Scholar]
- 8.Yen W-CC, et al. Direct growth of self-crystallized graphene and graphite nanoballs with Ni vapor-assisted growth: from controllable growth to material characterization. Sci. Rep. 2014;4:4739. doi: 10.1038/srep04739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murakami K, et al. Direct synthesis of large area graphene on insulating substrate by gallium vapor-assisted chemical vapor deposition. Appl. Phys. Lett. 2015;106:093112. doi: 10.1063/1.4914114. [DOI] [Google Scholar]
- 10.Chugh S, et al. Comparison of graphene growth on arbitrary non-catalytic substrates using low-temperature PECVD. Carbon. 2015;93:393–399. doi: 10.1016/j.carbon.2015.05.035. [DOI] [Google Scholar]
- 11.Ueno K, Ichikawa H, Uchida T. Effect of current stress during thermal CVD of multilayer graphene on cobalt catalytic layer. Jpn. J. Appl. Phys. 2016;55:04EC13. doi: 10.7567/JJAP.55.04EC13. [DOI] [Google Scholar]
- 12.Peng Z, Yan Z, Sun Z, Tour JM. Direct growth of bilayer graphene on SiO2 substrates by carbon diffusion through nickel. ACS Nano. 2011;5:8241–8247. doi: 10.1021/nn202923y. [DOI] [PubMed] [Google Scholar]
- 13.Kwak J, et al. Near room-temperature synthesis of transfer-free graphene films. Nat. Commun. 2012;3:645. doi: 10.1038/ncomms1650. [DOI] [PubMed] [Google Scholar]
- 14.Banno K, et al. Transfer-free graphene synthesis on insulating substrates via agglomeration phenomena of catalytic nickel films. Appl. Phys. Lett. 2013;103:082112. doi: 10.1063/1.4818342. [DOI] [Google Scholar]
- 15.Xiong W, et al. Solid-state graphene formation via a nickel carbide intermediate phase. RSC Adv. 2015;5:99037–99043. doi: 10.1039/C5RA18682J. [DOI] [Google Scholar]
- 16.Berman D, et al. Metal-induced rapid transformation of diamond into single and multilayer graphene on wafer scale. Nat. Commun. 2016;7:12099. doi: 10.1038/ncomms12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vishwakarma R, et al. Transfer free graphene growth on SiO2 substrate at 250 °C. Sci. Rep. 2017;7:43756. doi: 10.1038/srep43756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byun SJ, et al. Graphenes converted from polymers. J. Phys. Chem. Lett. 2011;2:493–497. doi: 10.1021/jz200001g. [DOI] [Google Scholar]
- 19.Gumi K, Ohno Y, Maehashi K, Inoue K, Matsumoto K. Direct Synthesis of Graphene on SiO2 Substrates by Transfer-Free Processes. Jpn. J. Appl. Phys. 2012;51:06FD12. doi: 10.7567/JJAP.51.06FD12. [DOI] [Google Scholar]
- 20.Weatherup RS, et al. Introducing carbon diffusion barriers for uniform, high-quality graphene growth from solid sources. Nano Lett. 2013;13:4624–4631. doi: 10.1021/nl401601x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamaoki M, Imaeda H, Kishimoto S, Mizutani T. Transfer-free fabrication of graphene field effect transistor arrays using solid-phase growth of graphene on a SiO2/Si substrate. Appl. Phys. Lett. 2013;103:183114. doi: 10.1063/1.4829137. [DOI] [Google Scholar]
- 22.Tanaka H, Obata S, Saiki K. Reduction of graphene oxide at the interface between a Ni layer and a SiO2 substrate. Carbon. 2013;59:472–478. doi: 10.1016/j.carbon.2013.03.041. [DOI] [Google Scholar]
- 23.Sato M, et al. Intercalated multilayer graphene wires and metal/multilayer graphene hybrid wires obtained by annealing sputtered amorphous carbon. Jpn. J. Appl. Phys. 2014;53:04EB05. doi: 10.7567/JJAP.53.04EB05. [DOI] [Google Scholar]
- 24.Kosaka M, Takano S, Hasegawa K, Noda S. Direct synthesis of few- and multi-layer graphene films on dielectric substrates by “etching-precipitation” method. Carbon. 2015;82:254–263. doi: 10.1016/j.carbon.2014.10.069. [DOI] [Google Scholar]
- 25.Zhuo QQ, et al. Transfer-free synthesis of doped and patterned graphene films. ACS Nano. 2015;9:594–601. doi: 10.1021/nn505913v. [DOI] [PubMed] [Google Scholar]
- 26.Yamada J, Ueda Y, Maruyama T, Naritsuka S. Direct growth of multilayer graphene by precipitation using W capping layer. Jpn. J. Appl. Phys. 2016;55:100302. doi: 10.7567/JJAP.55.100302. [DOI] [Google Scholar]
- 27.Nast O, Puzzer T, Koschier LM, Sproul AB, Wenham SR. Aluminum-induced crystallization of amorphous silicon on glass substrates above and below the eutectic temperature. Appl. Phys. Lett. 1998;73:3214–3216. doi: 10.1063/1.122722. [DOI] [Google Scholar]
- 28.Sarikov A, et al. A kinetic simulation study of the mechanisms of aluminum induced layer exchange process. J. Appl. Phys. 2010;107:114318. doi: 10.1063/1.3431385. [DOI] [Google Scholar]
- 29.Wang Z, Gu L, Jeurgens LPH, Phillipp F, Mittemeijer EJ. Real-time visualization of convective transportation of solid materials at nanoscale. Nano Lett. 2012;12:6126–6132. doi: 10.1021/nl303801u. [DOI] [PubMed] [Google Scholar]
- 30.Birajdar BI, Antesberger T, Butz B, Stutzmann M, Spiecker E. Direct in situ transmission electron microscopy observation of Al push up during early stages of the Al-induced layer exchange. Scr. Mater. 2012;66:550–553. doi: 10.1016/j.scriptamat.2011.12.045. [DOI] [Google Scholar]
- 31.Toko K, et al. Selective formation of large-grained, (100)- or (111)-oriented Si on glass by Al-induced layer exchange. J. Appl. Phys. 2014;115:094301. doi: 10.1063/1.4867218. [DOI] [Google Scholar]
- 32.Toko K, et al. Highly (111)-oriented Ge thin films on insulators formed by Al-induced crystallization. Appl. Phys. Lett. 2012;101:072106. doi: 10.1063/1.4744962. [DOI] [Google Scholar]
- 33.Park J-H, Kasahara K, Hamaya K, Miyao M, Sadoh T. High carrier mobility in orientation-controlled large-grain (≥50 μm) Ge directly formed on flexible plastic by nucleation-controlled gold-induced-crystallization. Appl. Phys. Lett. 2014;104:252110. doi: 10.1063/1.4885716. [DOI] [Google Scholar]
- 34.Toko K, et al. Low-temperature (180 °C) formation of large-grained Ge (111) thin film on insulator using accelerated metal-induced crystallization. Appl. Phys. Lett. 2014;104:022106. doi: 10.1063/1.4861890. [DOI] [Google Scholar]
- 35.Toko K, Nakazawa K, Saitoh N, Yoshizawa N, Suemasu T. Improved surface quality of the metal-induced crystallized Ge seed layer and its influence on subsequent epitaxy. Cryst. Growth Des. 2015;15:1535–1539. doi: 10.1021/acs.cgd.5b00060. [DOI] [Google Scholar]
- 36.Higashi H, et al. Electrical properties of pseudo-single-crystalline Ge films grown by Au-induced layer exchange crystallization at 250 °C. J. Appl. Phys. 2018;123:215704. doi: 10.1063/1.5031469. [DOI] [Google Scholar]
- 37.Yoshimine R, Toko K, Saitoh N, Yoshizawa N, Suemasu T. Silver-induced layer exchange for polycrystalline germanium on a flexible plastic substrate. J. Appl. Phys. 2017;122:215305. doi: 10.1063/1.5005002. [DOI] [Google Scholar]
- 38.Kurosawa M, Kawabata N, Sadoh T, Miyao M. Enhanced interfacial-nucleation in Al-induced crystallization for (111) oriented Si1–xGex (0 ≤ x ≤ 1) films on insulating substrates. ECS J. Solid State Sci. Technol. 2012;1:144–147. doi: 10.1149/2.010203jss. [DOI] [Google Scholar]
- 39.Toko K, Kusano K, Nakata M, Suemasu T. Low temperature synthesis of highly oriented p-type Si1−xGex (x: 0–1) on an insulator by Al-induced layer exchange. J. Appl. Phys. 2017;122:155305. doi: 10.1063/1.4996373. [DOI] [Google Scholar]
- 40.Murata H, Toko K, Suemasu T. Multilayer graphene on insulator formed by Co-induced layer exchange. Jpn. J. Appl. Phys. 2017;56:05DE03. doi: 10.7567/JJAP.56.05DE03. [DOI] [Google Scholar]
- 41.Murata H, Toko K, Saitoh N, Yoshizawa N, Suemasu T. Direct synthesis of multilayer graphene on an insulator by Ni-induced layer exchange growth of amorphous carbon. Appl. Phys. Lett. 2017;110:033108. doi: 10.1063/1.4974318. [DOI] [Google Scholar]
- 42.Murata H, Saitoh N, Yoshizawa N, Suemasu T, Toko K. High-quality multilayer graphene on an insulator formed by diffusion controlled Ni-induced layer exchange. Appl. Phys. Lett. 2017;111:243104. doi: 10.1063/1.5010982. [DOI] [Google Scholar]
- 43.Chu PK, Li L. Characterization of amorphous and nanocrystalline carbon films. Mater. Chem. Phys. 2006;96:253–277. doi: 10.1016/j.matchemphys.2005.07.048. [DOI] [Google Scholar]
- 44.Borysiuk J, et al. Role of structure of C-terminated 4H-SiC(000-1) surface in growth of graphene layers: Transmission electron microscopy and density functional theory studies. Phys. Rev. B. 2012;85:045426. doi: 10.1103/PhysRevB.85.045426. [DOI] [Google Scholar]
- 45.Borysiuk J, et al. Structural defects in epitaxial graphene layers synthesized on C-terminated 4H-SiC (000-1) surface-Transmission electron microscopy and density functional theory studies. J. Appl. Phys. 2014;115:054310. doi: 10.1063/1.4863644. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are available upon reasonable request from the corresponding author K.T. (toko@bk.tsukuba.ac.jp).