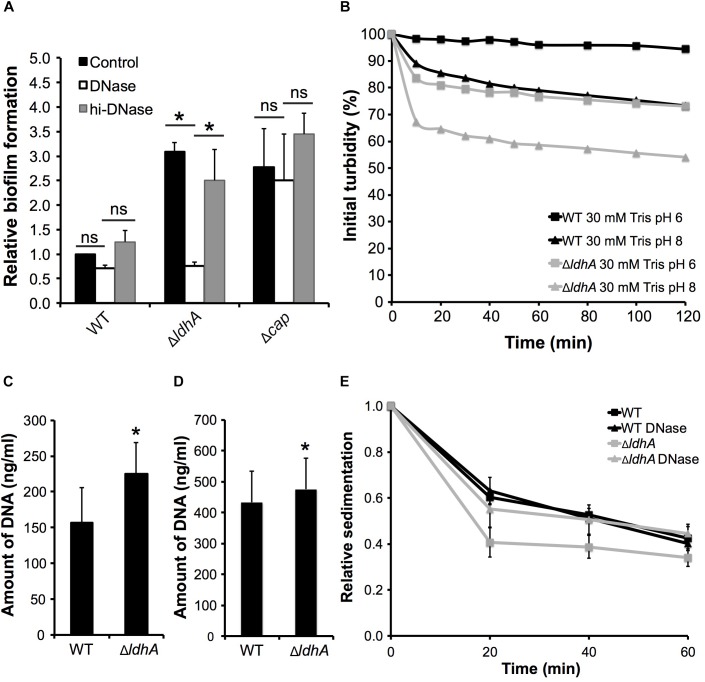
FIGURE 5.
Increased biofilm formation and aggregation by ΔldhA is associated with an increase in extracellular DNA and autolysis. (A) Biofilm formation by the wild-type, ΔldhA, and Δcap mutant strains in the presence or absence of DNase I. Δcap was used as a positive control. Bacteria were resuspended in GC liquid supplemented with 1% Kellogg’s with or without DNase I to OD600 of 0.05 and grown under static conditions for 24 h. Heat-treated DNase I was used as control (hi–DNase). Washed biofilms were stained with crystal violet and dissolved with acetic acid. The absorbance was measured at 630 nm. The experiment was performed three times in triplicate. (B) Autolysis under non-growth conditions. The wild-type and the ΔldhA strains were resuspended in GC liquid supplemented with 1% Kellogg’s, to an OD600 of 0.05 and grown for 3 h. Bacteria were centrifuged, washed with PBS, and resuspended in 30 mM Tris-HCL buffers at pH 6 or 8 to an OD600 of 1. Absorbance values were acquired every 10 min for the first hour and at 20 min intervals during the second hour. The values were used to calculate the percentage of initial turbidity. One representative experiment, performed in duplicate, out of four is shown. Detection of eDNA in the culture supernatants (C) and in biofilms (D) of the wild-type and ΔldhA strains using a fluorescence-based Quant-IT PicoGreen dsDNA assay kit. Experiments were performed three times in triplicate. (E) Sedimentation of wild-type and ΔldhA aggregates grown in the presence or absence of DNase I. Bacteria were grown for 3 h in the presence or absence of DNase I under shaking conditions at 37°C and 5% CO2 and then moved to static conditions at room temperature. The absorbance (OD600) of the top layer of the culture was measured every 20 min. Data are presented as relative values compared to the 0 min time point (set to 1). Experiments were performed three times. Unless stated, the bars represent the means, with error bars representing the standard deviations. ∗p < 0.05. ns, non-significant.