Abstract
Cyanotic congenital heart disease (CCHD), a term describing the most severe congenital heart diseases are characterized by the anatomic malformation of a right to left shunt. Although the incidence of CCHD are far less than the that of congenital heart diseases (CHD), patients with CCHD always present severe clinical features such as hypoxia, dyspnea, and heart failure. Chronic hypoxia induces hypoxemia that significantly contributes to poor prognosis in CCHD. Current studies have demonstrated that the prolyl-4-hydroxylase2 (PHD2, encoded by EGLN1)/hypoxia-inducible factor-1A (HIF-1A) pathway is a key regulator of hypoxic response. Thus, we aim to assess the associations of single polymorphisms (SNPs) of the EGLN1 gene and hypoxic response in CCHD. A missense variant of EGLN1 c.380G>C (rs1209790) was found in 46 patients (46/126), with lower hypoxia incidence and higher rate of collateral vessel formation, compared with the wild type (P < 0.05). In vitro experiments, during hypoxia, EGLN1 mutation reduced EGLN1 expression compared with the wild type, with higher HIF-1A, VEGF and EPO expression levels in the mutant. No difference in HK1 expression was observed between the mutant and wild type. CCHD patients with c.380G>C showed improved response to hypoxia compared with the wild-type counterparts. The EGLN1 c.380G>C mutation improves hypoxic response through the PHD2/HIF-1A pathway, which may provide a molecular mechanism for hypoxic response in CCHD. The effects of the EGLN1 c.380G>C mutation on CCHD prognosis deserve further investigation.
Keywords: Cyanotic congenital heart disease, EGLN1, Hypoxic response, Mutation, Polymorphism
Abbreviations: HIF-1A, hypoxia inducible factor-1A; VEGF, vascular endothelial growth factor; EPO, erythropoietin; HK1, Hexokinase I
Introduction
Cyanotic congenital heart disease (CCHD), a term describing the most severe congenital heart diseases characterized by the anatomic malformation of a right to left shunt, includes tetralogy of Fallot (TOF), double outlet of right ventricle (DORV), transposition of the great arteries (TGA), and pulmonary artery (PA) defects. Although only about 0.08–0.12% of live births are affected by CCHD, far less than the incidence of congenital heart diseases (CHD), patients with CCHD always present severe clinical features such as hypoxia, dyspnea, and heart failure.1, 2, 3 Many patients present cyanosis before they could benefit from surgical treatment.4
Based on the specific anatomic malformation, patients with CCHD usually develop chronic hypoxia, which induces hypoxemia that significantly contributes to poor prognosis in CCHD, with dysplasia, convulsions, and sudden death, even after an urgent surgical intervention.5, 6, 7 Consequently, the ability of hypoxic response determines the prognosis of CCHD patients. Current studies have demonstrated that the prolyl-4-hydroxylase2 (PHD2, encoded by EGLN1)/hypoxia-inducible factor-1A (HIF-1A) pathway is a key regulator of hypoxic response. Under normoxia, PHD2 triggers the oxygen-dependent hydroxylation of ODD within HIF-1A. During hypoxia, however, such hydroxylation is blocked, resulting in HIF-1A accumulation, angiogenesis, erythropoiesis, and glycolysis. It was reported by many studies that angiogenesis and erythropoiesis enhance hypoxic response through the PHD2/HIF-1A pathway, improving cell survival under hypoxic conditions.8 These findings indicated that the PHD2/HIF-1A pathway plays a crucial role in hypoxic response in CCHD.
Lorenzo FR9 assessed EGLN1 polymorphisms in Tibetan high-altitude adaptation. However, most studies focused on polycythemia; indeed, more than 13 EGLN1 mutations have been reported in association with polycythemia.10, 11, 12, 13 Meanwhile, the effects of EGLN1 polymorphisms on CCHD remain unclear. Thus, we hypothesized that EGLN1 polymorphisms affect hypoxic response in CCHD through the PHD2/HIF-1A pathway.
This study aimed to assess the association of hypoxia with collateral vessel malformation among different EGLN1 genotypes. In addition, we analyzed the expression levels of target genes involved in the PHD2/HIF-1A pathway for various genotypes. The current findings may help further understand the genetic basis of hypoxic response, providing new options for intervention and adjunctive therapy aiming to improve hypoxic response in CCHD.
Materials and methods
Subjects
A total of 126 CCHD patients in Children's Hospital of Chongqing Medical University were screened for EGLN1 by sequencing (60 males and 66 females; 19.80 ± 1.99 months old). They included 58 TOF, 35 DORV, 10 PA defect, 8 persistent truncus arteriosus (PTA), 7 TGA, 6 anomalous pulmonary venous drainage, and 2 complete atrioventricular septal defect (CAVSD) cases. Mean variabilities of limb SpO2 were less than 2%. Hypoxia was defined as SpO2<90%, and normoxia as SpO2≥90%. Development of collateral vessel was observed by echocardiography during hospitalization. The participants without consanguineous marriages for at least three generations or a family history of congenital heart diseases were Chinese Han individuals. This study was approved by the local ethics committees of Children's Hospital of Chongqing Medical University. All patients provided signed written informed consent. The privacy rights of human subjects were observed.
Sample preparation and genomic analyses
DNA was extracted from peripheral blood with MagPure Blood DNA Mini KF Kit (Magen, NO:MD5111-01). The obtained DNA was submitted to high-throughput sequencing of the EGLN1 gene, including the 5′UTR and 3′UTR, exons, and the 2 kb region upstream the promoter. This work was completed by Beijing Genomics Institute. Then, the gene variants were identified by Sanger sequencing, with the DNA-Star software used for data analysis.
Cell culture
HEK-293 cells were cultured in Dulbecco's Eagle's Modified Media (DMEM, Gibco) containing 10% fetal bovine serum (FBS) (Gibco) and 100 mg/ml penicillin/streptomycin in a humidified environment with 21% O2 and 5% CO2, at 37 °C. HEK-293 cells were co-transfected with 1.8 μg total DNA (variant, wild-type, and vector, respectively) and 4.5 μl Lipofectamine 2000 (Invitrogen). For normoxic experiments, the cells were cultured in a humidified environment containing 21% O2 and 5% CO2 at 37 °C. Hypoxic experiments were performed by culture in presence of 3% O2 and 5% CO2, also at 37 °C.
Real-time PCR
A total of 48 h after transfection, total RNA was extracted with TRIzol Reagent (Ambion), and reverse-transcribed into complementary DNA (cDNA) using the Prime Script RT reagent kit containing gDNA Eraser (Takara, NO: RR047A). Then, cDNA was submitted to PCR amplification with primer sets for actin, HIF-1A, VEGF, EPO and HK1. Actin gene primers were a gift from Sangon Biotech. The other primers were: HIF-1A, forward 5′-TGGAGTGCAGTGGAGCAATC-3′ and reverse 5′-CAGGCATGGTGGTACATGCT-3′; VEGF, forward 5′-GGTCCCTCTTGGAATTGGAT-3′ and reverse 5′-TGTATGTGGGTGGGTGTGTC-3′; EPO, forward 5′-CTTCTCCTGTCCCTGCTGTC-3′ and reverse 5′-TGATATTCTCGGCCTCCTTG-3′; HK1 forward 5′-TCCTCGTCAAGACAGTGTGC-3′ and reverse 5′-CCCACAGTCACATTCAGACG -3′.
Immunoblotting
A total of 52 h after transfection, total cellular protein was extracted on ice using 1 × lysis buffer (KeyGEN BioTECH, NO: KGP250) supplemented with a protease inhibitor and a phosphatase inhibitor. Equal amounts of protein were resolved by 10% SDS-polyacrylamide gel electrophoresis and transferred onto PVDF membranes. Non-specific bands were blocked with TBS-T containing 5% nonfat-dried milk for 1 h. Then, the membranes were successively incubated with specific primary antibodies at 4 °C for 15 h, and secondary antibodies at room temperature for 1.5 h. The membranes were washed three times with TBS-T, and target protein bands were visualized by ECL (KeyGEN BioTECH, NO: KGP1127). The primary antibodies used were: rabbit anti-EGLN1 mAb (Cell Signaling Technology,NO:4835S, 1:1000), rabbit anti—HIF—1 alpha mAb (Cell Signaling Technology,NO:14179S,1:500), anti-VEGF antibody (Arigo,NO:10513,1:500), anti-EPO antibody (ab126876, 1:500), rabbit anti-hexokinase I mAb (Cell Signaling Technology, NO:2024S), and anti-actin antibody (4A Biotech Co,NO:ICM001-050,1:1000), Secondary antibodies were goat anti-mouse IgG (ATGene)and goat anti-Rabbit IgG (Sino biological Inc,NO:HO10AP0801).
Statistics
SPSS 20.0 was used for statistical analyses. Data are mean ± standard error (SE). Enumeration data were assessed the Chi-square test. Differences among groups were analyzed by one way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results
Genomic testing for hypoxic adaptation related mutations in patients with CCHD
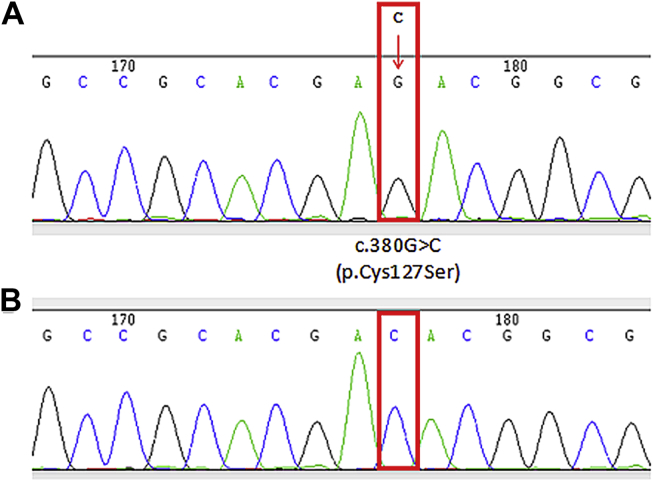
The missense mutation c.380G>C was found in the EGLN1 gene in 46 patients with CCHD (21 males and 25 females; variant and wild-type bearing patients were 22.82 ± 3.89 months and 18.06 ± 2.20 months old, respectively; Fig. 1A and B). A total of 36.96% (17/46) of these carriers had hypoxemia, indicating a lower rate compared with the wild-type group (52/80) (p = 0.003, Table 1). In addition, angiogenesis is known to benefit hypoxic response. Thus, to further assess the association of c.380G>C mutation with hypoxic response, collateral vessel formation was observed. Interestingly, 50% (23/46) of mutation carriers showed collateral vessel formation, a higher rate compared with that of the wild-type group (22/80) (p = 0.01, Table 1). Collateral vessel formation is a compensatory mechanism for response to the hypoxic environment. In this study, high incidence of collateral vessel formation was observed in patients with the c.380G>C mutation, suggesting this gene alteration may enhance hypoxic response.
Figure 1.
Identification of EGLN1 mutation in patients with cyanotic congenital heart disease. Results are presented with template strand which is opposite to the coding strand, so the remarked sign is changing C into G. (A). Sequencing results of EGLN1 c.380G>C mutation in 46 patients. (B) Sequencing results of wild-type DNA mutation in 80 patients.
Table 1.
Associations of EGLN1 gene c.380G>C mutation with CCHD features.
| parameter | Total (n = 126) | c.380G>C mutation (n = 46) | Wild-type (n = 80) | P-value |
|---|---|---|---|---|
| Hypoxia, n (%) | 69 (54.76%) | 17 (36.96%) | 52 (65.00%) | 0.003 |
| CV, n (%) | 45 (35.71%) | 23 (50.00%) | 22 (7.50%) | 0.01 |
CV: collateral vessel.
Plasmid validation and transfection
Recombinant plasmids (c.380G>C mutation, wild-type, and vector) were confirmed by sequencing. A total of 48 h after transfection, GFP expression by the plasmid vector was observed on a fluorescence microscope, suggesting successful transfection.
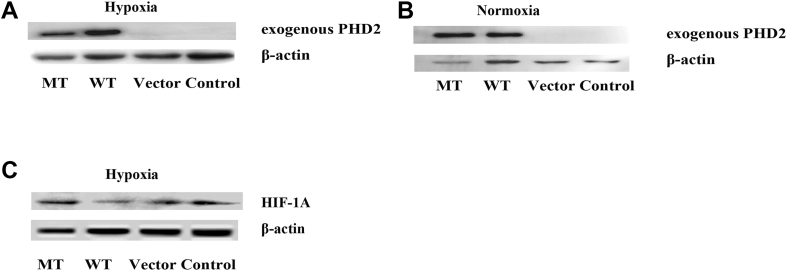
The c.380G>C mutation reduces PHD2 protein expression under hypoxic conditions
To assess the effect of the c.380G>C mutation, PHD2 protein expression was analyzed by immunoblot. Unlike the empty vector and control (untreated cells) groups, the wild-type and c.380G>C mutation groups expressed the exogenous PHD2 protein, providing further evidence for successful transfection and encoding. As shown in Fig. 2A, PHD2 levels in the c.380G>C mutation group decreased by 53.34% compared with the wild-type group under hypoxic conditions (p < 0.05, Fig. 2A). However, there was no significant difference between the c.380G>C mutation and wild type groups in normoxia (P > 0.05,Fig. 2B).
Figure 2.
Functional study of EGLN1 mutation using western blot. Expression of exogenous PHD2 protein (encoded by EGLN1) was observed only in c.380G>C mutation and wild-type, providing a further evidence for the successful transfection. (A) Cells were transfected in a 6-well format with 1.8ug recombinant plasmids under hypoxic conditions (3% O2). c.380G>C mutation reduced the expression of PHD2 compared with wild-type under hypoxic conditions. β-actin was used as a loading control. (B) There is no significant difference of PHD2 protein expression between c.380G>C mutation and wild-type during normoxia. (C) Expression of HIF-1A in cells transfected with recombinant plasmids under hypoxic condition. Cells were infected with c.380G>C mutation, wild-type and empty vector. Under hypoxic conditions, increased expression levels of HIF-1A in c.380G>C mutation were observed in four independent experiments. MT: c.380G>C mutation; WT: wild-type; vector: empty vector with no PHD2 expression; control: untreated group.
The c.380G>C mutation activates the PHD2/HIF-1A pathway and induces HIF-1A accumulation during hypoxia
PHD2 is a hydroxylase that interacts with HIF-1A and hydroxylates its ODD region, resulting in acute degradation of the HIF-1A protein. Thus, we hypothesized that the c.380G>C mutation may affect the degradation of the HIF-1A protein by altering PHD2 mediated hydroxylation. To test this hypothesis, HIF-1A expression was analyzed in cells transfected with different recombinant plasmids. Under hypoxic conditions, HIF-1A protein expression levels in the c.380G>C mutation group were overtly increased (by 1.81-fold) compared with the wild-type group (P < 0.01, Fig. 2C). These results suggested that the c.380G>C mutation may activate the PHD2/HIF-1A pathway and induce HIF-1A protein expression. Under normoxic conditions, HIF-1A protein was degraded immediately after production. Thus, the HIF-1A protein under normoxic conditions was barely detectable. In addition, there were no significant differences among groups under hypoxic and normoxic conditions (data not shown).
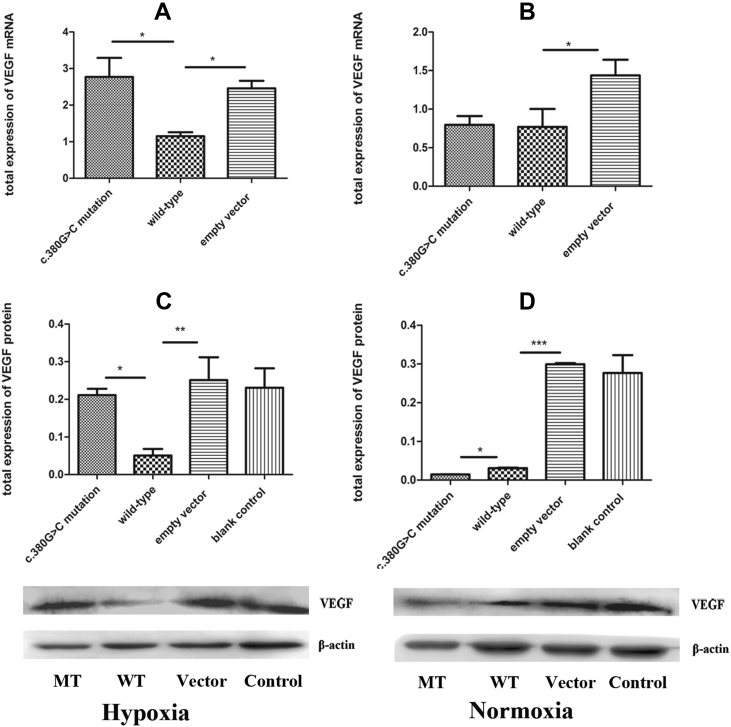
The c.380G>C mutation stimulates VEGF expression in association with the PHD2/HIF-1A pathway under hypoxic conditions
VEGF, a crucial component of the PHD2/HIF-1A pathway, is induced by the HIF-1A protein through PHD2/HIF-1A signaling under hypoxic conditions. Meanwhile, it was shown that the c.380G>C mutation induces HIF-1A protein expression. Hence, it is conceivable that the c.380G>C mutation may upregulate VEGF in association with the PHD2/HIF-1A pathway under hypoxic conditions. Indeed, our results showed that VEGF mRNA levels in the c.380G>C mutation group were higher (1.41-fold) compared with the wild-type group in hypoxia (p < 0.05, Fig. 3A); the VEGF protein was also up-regulated by 3.19-fold (p < 0.05, Fig. 3C). However, these beneficial effects were not observed in normoxia (p > 0.05, Fig. 4B; p < 0.05, Fig. 3B and D). These findings indicated that the c.380G>C mutation upregulated VEGF in association with the PHD2/HIF-1A pathway in hypoxia.
Figure 3.
Expression of VEGF in cells transfected with c.380G>C mutation, wild-type and empty vector. (A) Under hypoxic conditions, increased expression levels of VEGF mRNA in c.380G>C mutation were observed compared with wild-type. (B) No significant difference of VEGF mRNA expression was observed between c.380G>C mutation and wild-type during normoxia. (C) Under hypoxic conditions, increased expression levels of VEGF protein in c.380G>C mutation were observed compared with wild-type. (D) Under normoxic conditions, expression of VEGF protein in c.380G>C mutation is lower than that in wild-type. MT: c.380G>C mutation; WT: wild-type; vector: empty vector with no PHD2 expression; control: untreated group.
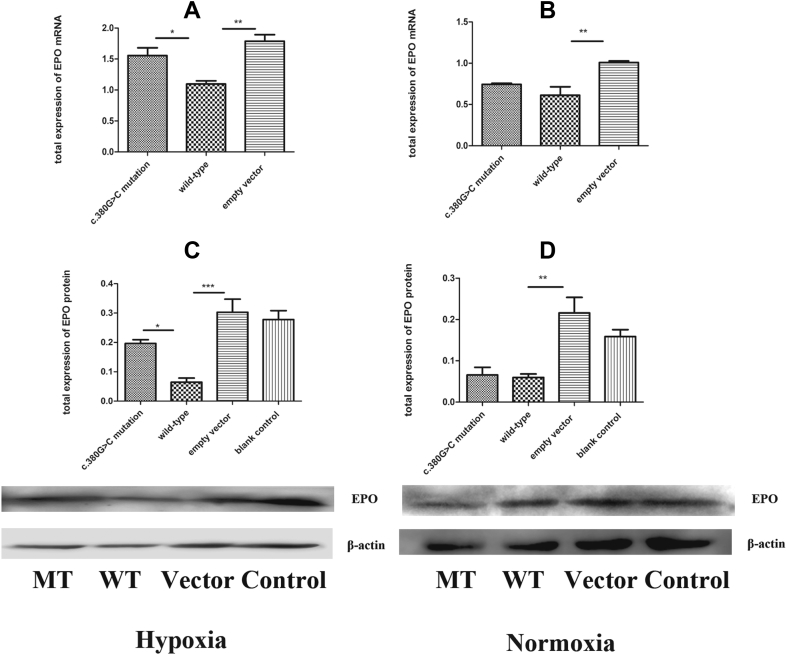
Figure 4.
Expression of EPO in cells transfected with c.380G>C mutation, wild-type and empty vector. (A) Under hypoxic conditions, increased expression levels of EPO mRNA in c.380G>C mutation were observed compared with wild-type. (B) No significant difference of EPO mRNA expression between c.380G>C mutation and wild-type was observed during normoxia. (C) Under hypoxic conditions, increased expression level of EPO protein in c.380G>C mutation were observed compared with wild-type. (D) Under normoxic conditions, expression of EPO protein in c.380G>C mutation is lower than that in wild-type. MT: c.380G>C mutation; WT: wild-type; vector: empty vector with no PHD2 expression; control: untreated group.
The c.380G>C mutation increases EPO expression in association with PHD2/HIF-1A signaling under hypoxic conditions
EPO, another critical component of the PHD2/HIF-1A pathway, is upregulated by HIF-1A in hypoxia. Next, EPO expression levels were detected by immunoblot and RT-PCR, respectively, to further assess the effects of the c.380G>C mutation on hypoxic response associated with PHD2/HIF-1A signaling. As expected, the gene expression levels of EPO were increased by 0.42-fold in c.380G>C mutation harboring cells versus wild-type counterparts (p < 0.05, Fig. 4A). Similarly, EPO protein levels in the c.380G>C mutation group were significantly increased by 2.05-fold compared with wild-type counterparts (p = 0.011, Fig. 4C). Next, EPO expression in normoxic conditions was analyzed, and upregulation was not observed (p > 0.05, Fig. 4B; p > 0.05, Fig. 4D). These findings suggested that the c.380G>C mutation increased EPO expression in association with the PHD2/HIF-1A pathway during hypoxia.
HK1 expression is associated with the PHD2/HIF-1A pathway
It is well-known that hypoxia stimulates glycolysis. Next, to comprehensively assess the effects of the c.380G>C mutation on glycolysis associated with the PHD2/HIF-1A pathway, the expression levels of HK1, a critical enzyme involved in glycolysis, were determined by Western blot and RT-PCR, respectively. No significant differences were observed among all groups under hypoxic or normoxic conditions (data not shown).
Discussion
This study revealed a missense variant of the EGLN1 gene (c.380G>C) in patients with CCHD, with lower hypoxia incidence in CCHD patients with the c.380G>C mutation compared with wild-type subjects. In addition, the incidence of collateral vessel formation, considered a critical mechanism of hypoxic response, was higher in the c.380G>C mutation group compared with wild-type patients. Next, in vitro experiments were performed to assess the effects of the c.380G>C mutation on hypoxic response. Our results demonstrated that the c.380G>C mutation reduced PHD2 protein expression, which resulted in alleviated hydroxylation mediated by PHD2 and increased accumulation of the HIF-1A protein. VEGF and EPO are crucial components of the PHD2/HIF-1A pathway. Many studies have reported that PHD2 alteration indeed affects VEGF and EPO expression. In this study, the c.380G>C mutation upregulated HIF-1A, VEGF, and EPO in association with the PHD2/HIF-1A pathway under hypoxic conditions.
The PHD2/HIF-1A pathway in the pathogenesis of hypoxemia and CCHD have been described by many experimental and clinical studies.14, 15 Under hypoxic conditions, upregulating the adaptive gene (e.g. VEGF, EPO, and several glycolytic enzymes) by the accumulated HIF-1A protein plays an important role in response to hypoxia, and improves the outcome of CCHD patients.16, 17, 18, 19, 20 Overexpression of VEGF increases neovascularization and peripheral oxygen delivery, improving hypoxic response in individuals with hypoxemia.17, 21 Likewise, EPO maintains oxygen homeostasis by stimulating the proliferation of erythroid progenitors for erythropoiesis under hypoxic conditions. Increased erythrocyte amounts facilitate O2 transport in response to hypoxia.22 These evidences demonstrate that overexpression of VEGF and EPO indeed could improve hypoxemia and CCHD prognosis in association with the PHD2/HIF-1A pathway. In this study, compared with wild-type individuals, the c.380G>C group showed a lower hypoxia incidence and a higher collateral vessel formation rate. Angiogenesis is one of the known mechanisms of hypoxia response. The current findings suggested that the c.380G>C mutation might benefit hypoxic response. In in vitro experiments, we found that the c.380G>C mutation increased HIF-1A protein accumulation. In addition VEGF and EPO were upregulated in the c.380G>C mutation group, in association with PHD2/HIF-1A signaling. Overexpression of VEGF and EPO enhances tolerance to hypoxia and improves the outcome of CCHD patients, as elucidated previously. Therefore, our findings suggested that the c.380G>C mutation protects patients with CCHD from hypoxia in association with the PHD2/HIF-1A pathway. This may provide a molecular mechanism for hypoxic response in patients with CCHD.
Although the c.380G>C mutation has been assessed for Tibetan high-altitude adaptation, its function has not been analyzed alone. On the one hand, Lorenzo FR9 analyzed the two missense variants c. [12C > G; 380G > C] together. On the other hand, the molecular mechanisms and pathophysiology of hypoxic response between CCHD and Tibetan may be different. We first assessed the association of the c.380G>C mutation with CCHD, also analyzing the function of the c.380G>C mutation in vitro. Recently, associations of gene polymorphisms with diseases have been explored in many studies, with significant findings reported. Indeed, gene polymorphisms play important roles in many diseases, e.g. effects of R530X MIB1 on left ventricular non-compaction (LVNC),23 c.300delA DNAJC19 on dilated cardiomyopathy syndrome (DCMA),24 c.609C > G EGLN1 on polycythemia,12 et al. However, the associations of gene polymorphisms with CCHD remain unclear. Polymorphisms in EGLN1 were reported in polycythemia and high-altitude adaptation. The associations of EGLN1 polymorphisms with CCHD remain undefined. Thus, this may be the first study assessing the effects of EGLN1 gene polymorphisms on hypoxic response in CCHD. Our findings may help further understand the genetic basis of hypoxic response, and provide insights for developing new approaches in reducing CCHD mortality, improving patient prognosis, earning more time before surgery, and improving the patients' quality of life.
Following hypoxia, the HIF-1A protein accumulates at an alarming rate, but is rapidly degraded by a mechanism involving PHD2. PHD2 protein levels directly affect HIF-1A expression by controlling the hydroxylation of ODD within HIF-1A, but not transcription. Thus, PHD2 expression does not alter the gene expression of HIF-1A, which fully accounts for the findings of no significant differences in HIF-1A mRNA expression among groups. Another characteristic of response to chronic hypoxia is a shift from lipid oxidation to glycolysis. As a critical glycolytic enzyme, HK1 is upregulated under hypoxic conditions.25 Surprisingly, discordant changes were found in the expression levels of HK1 and HIF-1A. HK1 expression showed no significant difference between the c.380G>C mutation and wild-type groups. This may be explained by the fact that HK1 is regulated by the AMP-dependent protein kinase (AMPK) signaling pathway, not PHD2/HIF-1A signaling.25, 26 Inevitably, this study had several limitations, including small sample size, which also limited sub-group analysis by severity of congenital heart defects.
Conclusion
Hypoxemia incidence in CCHD patients with the c.380G>C mutation in EGLN1 is lower than in wild type counterparts. Meanwhile, the c.380G>C mutation contributes to collateral vessel formation. The c.380G>C upregulates VEGF and EPO in association with the PHD2/HIF-1A pathway to improve hypoxic response in patients with CCHD. This study may provide a new approach for intervention and adjunctive therapy to improve hypoxic response in CCHD.
Conflict of interest
There are no financial or other interests of conflict exist.
Acknowledgements
This work is supported by the National Nature Science Foundation of China (81570218); Major Project of Chongqing Municipal Health Bureau (56-20141009)and clinical project of Children's Hospital of Chongqing Medical University (hjyn2012-6).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Hoffman J.I., Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell S.C., Korones S.B., Berendes H.W. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation. 1971;43(3):323–332. doi: 10.1161/01.cir.43.3.323. [DOI] [PubMed] [Google Scholar]
- 3.Chang R.K., Gurvitz M., Rodriguez S. Missed diagnosis of critical congenital heart disease. Arch Pediatr Adolesc Med. 2008;162(10):969–974. doi: 10.1001/archpedi.162.10.969. [DOI] [PubMed] [Google Scholar]
- 4.Syamasundar Rao P. Diagnosis and management of cyanotic congenital heart disease: part II. Indian J Pediatr. 2009;76(3):297–308. doi: 10.1007/s12098-009-0056-7. [DOI] [PubMed] [Google Scholar]
- 5.Zucker E.J., Koning J.L., Lee E.Y. Cyanotic congenital heart disease: essential primer for the practicing radiologist. Radiol Clin North Am. 2017;55(4):693–716. doi: 10.1016/j.rcl.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Cordina R.L., Celermajer D.S. Chronic cyanosis and vascular function: implications for patients with cyanotic congenital heart disease. Cardiol Young. 2010;20(3):242–253. doi: 10.1017/S1047951110000466. [DOI] [PubMed] [Google Scholar]
- 7.Rohit M., Shrivastava S. 2017 Sep 30. Acyanotic and cyanotic congenital heart diseases. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Bigham A.W., Lee F.S. Human high-altitude adaptation: forward genetics meets the HIF pathway. Genes Dev. 2014;28(20):2189–2204. doi: 10.1101/gad.250167.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorenzo F.R., Huff C., Myllymaki M. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet. 2014;46(9):951–956. doi: 10.1038/ng.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreuger J., Phillipson M. Targeting vascular and leukocyte communication in angiogenesis inflammation and fibrosis. Nat Rev Drug Discov. 2016;15(2):125–142. doi: 10.1038/nrd.2015.2. [DOI] [PubMed] [Google Scholar]
- 11.Ladroue C., Hoogewijs D., Gad S. Distinct deregulation of the hypoxia inducible factor by PHD2 mutants identified in germline DNA of patients with polycythemia. Haematologica. 2012;97(1):9–14. doi: 10.3324/haematol.2011.044644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albiero E., Ruggeri M., Fortuna S. Isolated erythrocytosis: study of 67 patients and identification of three novel germ-line mutations in the prolyl hydroxylase domain protein 2 (PHD2) gene. Haematologica. 2012;97(1):123–127. doi: 10.3324/haematol.2010.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bento C., Almeida H., Maia T.M. Molecular study of congenital erythrocytosis in 70 unrelated patients revealed a potential causal mutation in less than half of the cases (Where is/are the missing gene(s)?) Eur J Haematol. 2013;91(4):361–368. doi: 10.1111/ejh.12170. [DOI] [PubMed] [Google Scholar]
- 14.Semenza G.L. Hypoxia-inducible factor 1 and the molecular physiology of oxygen homeostasis. J Lab Clin Med. 1998;131(3):207–214. doi: 10.1016/s0022-2143(98)90091-9. [DOI] [PubMed] [Google Scholar]
- 15.Rajatapiti P., van der Horst I.W., de Rooij J.D. Expression of hypoxia-inducible factors in normal human lung development. Pediatr Dev Pathol. 2008;11(3):193–199. doi: 10.2350/07-04-0257.1. [DOI] [PubMed] [Google Scholar]
- 16.Lemus-Varela M.L., Flores-Soto M.E., Cervantes-Munguia R. Expression of HIF-1 alpha, VEGF and EPO in peripheral blood from patients with two cardiac abnormalities associated with hypoxia. Clin Biochem. 2010;43(3):234–239. doi: 10.1016/j.clinbiochem.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 17.El Hasnaoui-Saadani R., Marchant D., Pichon A. Epo deficiency alters cardiac adaptation to chronic hypoxia. Respir Physiol Neurobiol. 2013;186(2):146–154. doi: 10.1016/j.resp.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z.L., Wu Z.S., Hu J.G. Correlation of serum levels of VEGF and SDF-1 with the number and function of circulating EPCs in children with cyanotic congenital heart disease. Zhong Guo Dang Dai Er Ke Za Zhi. 2009;11(4):267–272. [PubMed] [Google Scholar]
- 19.Bielecka Z.F., Czarnecka A.M., Solarek W. Mechanisms of acquired resistance to tyrosine kinase inhibitors in clear - cell renal cell carcinoma (ccRCC) Curr Signal Transduct Ther. 2014;8(3):218–228. doi: 10.2174/1574362409666140206223014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin C., Zhou S., Xiao Y. Erythropoietin enhances mitochondrial biogenesis in cardiomyocytes exposed to chronic hypoxia through Akt/eNOS signalling pathway. Cell Biol Int. 2014;38(3):335–342. doi: 10.1002/cbin.10205. [DOI] [PubMed] [Google Scholar]
- 21.Avivi A., Resnick M.B., Nevo E. Adaptive hypoxic tolerance in the subterranean mole rat Spalax ehrenbergi: the role of vascular endothelial growth factor. FEBS Lett. 1999;452(3):133–140. doi: 10.1016/s0014-5793(99)00584-0. [DOI] [PubMed] [Google Scholar]
- 22.Arieli R., Heth G., Nevo E. Hematocrit and hemoglobin concentration in four chromosomal species and some isolated populations of actively speciating subterranean mole rats in Israel. Experientia. 1986;42(4):441–443. doi: 10.1007/BF02118650. [DOI] [PubMed] [Google Scholar]
- 23.Luxan G., Casanova J.C., Martinez-Poveda B. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat Med. 2013;19(2):193–201. doi: 10.1038/nm.3046. [DOI] [PubMed] [Google Scholar]
- 24.Ojala T., Polinati P., Manninen T. New mutation of mitochondrial DNAJC19 causing dilated and noncompaction cardiomyopathy, anemia, ataxia, and male genital anomalies. Pediatr Res. 2012;72(4):432–437. doi: 10.1038/pr.2012.92. [DOI] [PubMed] [Google Scholar]
- 25.Waskova-Arnostova P., Kasparova D., Elsnicova B. Chronic hypoxia enhances expression and activity of mitochondrial creatine kinase and hexokinase in the rat ventricular myocardium. Cell Physiol Biochem. 2014;33(2):310–320. doi: 10.1159/000356671. [DOI] [PubMed] [Google Scholar]
- 26.Holmes B.F., Kurth-Kraczek E.J., Winder W.W. Chronic activation of 5'-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87(5):1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]