Abstract
Acute brain changes are expected after concussion, yet there is growing evidence of persistent abnormalities well beyond clinical recovery and clearance to return to play. Multiparametric MRI is a powerful approach to non-invasively study structure-function relationships in the brain, however it remains challenging to interpret the complex and heterogeneous cascade of brain changes that manifest after concussion. Emerging conjunctive, data-driven analysis approaches like linked independent component analysis can integrate structural and functional imaging data to produce linked components that describe the shared inter-subject variance across images. These linked components not only offer the potential of a more comprehensive understanding of the underlying neurobiology of concussion, but can also provide reliable information at the level of an individual athlete. In this study, we analyzed resting-state functional MRI (rs-fMRI) and diffusion tensor imaging (DTI) within a cohort of female varsity rugby players (n = 52) through the in- and off-season, including concussed athletes (n = 21) who were studied longitudinally at three days, three months and six months after a diagnosed concussion. Linked components representing co-varying white matter microstructure and functional network connectivity characterized (a) the brain's acute response to concussion and (b) persistent alterations beyond clinical recovery. Furthermore, we demonstrate that these long-term brain changes related to specific aspects of a concussion history and allowed us to monitor individual athletes before and longitudinally after a diagnosed concussion.
Keywords: Functional MRI, Resting state connectivity, Diffusion weighted imaging, Concussion, Linked independent component analysis, Mild traumatic brain injury
Highlights
-
•
Diffusion alterations were present acutely post-concussion and during the in-season.
-
•
Linked variation of brain structure-function persisted 6-months after injury.
-
•
Multiparametric signatures related to concussion history, even in healthy athletes.
1. Introduction
Concussion is a prevalent injury for young athletes participating in contact sports and it provokes an acute set of heterogeneous symptoms that generally recover quickly. However, the underlying changes in the brain that are responsible for these symptoms and the long-term consequences of concussion remain elusive. There is a critical need to understand the physiological changes during recovery and the potentially lasting effects of early and/or multiple concussions (Kamins et al. 2017).
Diffusion tensor imaging (DTI) and resting-state functional MRI (rs-fMRI) are sensitive to different attributes (i.e. structure and functional) of the neurobiological cascade following concussion (Bigler and Maxwell 2012), a cascade that appears to persist beyond clinical recovery and clearance to return to play (Churchill et al. 2017). Traditionally, each MRI metric is analyzed separately and related to each other post-hoc. Such approaches fail to take advantage of the intertwined structure-function relationship in the brain and typically provide limited understanding of an individual longitudinally. A data-driven analysis that examines structure-function relationships jointly could yield powerful insights into the underlying neurobiological changes in young concussed athletes over time (Shin et al. 2017).
Linked independent component analysis (LICA) is a data-driven approach that evaluates shared inter-subject variations across the different MRI measures and provides a more coherent appreciation of the underlying structure-function modes of variation (Groves et al. 2011; Sui et al. 2012). LICA is a powerful approach that decomposes multiparametric imaging data in an unbiased manner to produce components of (a) spatial maps that show where each of the image-derived metrics of interest (i.e. DTI parameters, rs-fMRI network (RSN) connectivity) co-vary together across subjects and (b) common component weights. Component weights represent to what extent an individual subject expresses a linked component at a particular time. When investigating longitudinal multiparametric data this analysis approach may allow a more complete picture of how an individual concussed athlete's brain measures evolve together throughout recovery.
Here we examined co-varying changes in the brain's structure (DTI)-function (rs-fMRI) relationships in a cohort of female varsity rugby players over a five-year period. The MRI data from non-concussed rugby players throughout the in- and off-season were compared to concussed athletes acutely (24–72 h) and longitudinally (3-months and 6-months) post-concussion. We hypothesized that linked rs-fMRI and DTI components would characterize acute neurobiological mechanisms post-concussion and that there would be distinct brain abnormalities that persist beyond clinical and symptomatic recovery. Given the existing literature (Alosco et al. 2017), we predicted that the expression of these linked components would relate to concussion history. Furthermore, because this approach interrogates the imaging structure-function data together, it may provide a more reliable way to monitor individual concussed athletes longitudinally.
2. Methods
2.1. Patient recruitment and ethics approval
This study was approved by the University of Western Ontario's Health Sciences Research Ethics Board. Informed consent was obtained prior to the start of each season. All female rugby athletes on the varsity team were invited to participate and only sports-related concussions were enrolled in the longitudinal post-concussion protocol. Exclusion criteria included a previous concussion within 6-months of data collection, pre-existing systemic diseases or MRI exclusion criteria (metal fragments, implanted devices, severe heart disease, non-removable body piercings, or tattoos containing metallic inks). This longitudinal study followed the women's rugby team (ages 18–22, mean age [SD] = 20.1[1]) over a five-year period of national competition. Participants did not partake in all seasons. Table 1 details the activities during the season. We gathered 74 in-season and 63 off-season data sets that included returning players without a diagnosed concussion within the past 6-months. The in-season data was acquired during a high intensity training camp involving multiple daily contact practices and games as the participants strived to make the team. The off-season data was acquired in the winter term, 2–3 months after the regular season ended (approximately 6-months after the in-season scan). Players with a diagnosed concussion (n = 21) were assessed 24–72 h, 3-months and 6-months post-concussion.
Table 1.
Player activities throughout a regular varsity rugby season.
| Time period | Contact practices | Weight training | Cardio | Games | Notes |
|---|---|---|---|---|---|
| August (in-season) | 2/day | – | – | 3–4/week | 2-week in-season training camp |
| Sept-Oct | 4/week | 1/week | – | 1/week | Regular season |
| November | – | – | – | 1/day during tournament | National Championships (4 days) |
| Dec-Apr (off-season) | 1/week (technical skills, light contact compared to regular season practices) | 3–4/week | 3–4 /week | 1/month | Off-season with a 1 day tournament per month (Jan-Mar) |
2.2. Clinical assessment
Players suspected of a self-reported concussion were assessed at the Fowler Kennedy Sports Medicine Clinic within 24–72 h of their injury where an experienced Sports Medicine physician diagnosed a concussion based on a mechanism of injury consistent with TBI and Sport Concussion Assessment Tool (version 3) measures. SCAT3 was performed in accordance with the instructional guidelines (“SCAT3.,” 2013). The SCAT3 is composed of the Glasgow coma scale (GCS), the Maddocks Score and a cognitive and physical evaluation to evaluate the athlete's orientation, concentration, balance and coordination. The GCS assesses an athlete's eye, verbal, and motor responses and the Maddocks score consists of 5 questions to assess short-term memory. The SCAT3 also scores 22 possible symptoms (symptom score) on a scale from 0 to 6 (symptom severity score). We investigated the relationship between our MRI results with the SCAT3 self-reported symptoms and severity as these two measures were the only measures significantly different acutely post-concussion reported in a previous study of these athletes (Schranz et al. 2017). We also documented the time since the most recent concussion (in months) and the self-reported total number of concussions in their history and included previous concussions as a predictor of neuroimaging changes post-concussion (Table A.1).
2.3. MRI acquisition
All MRI data was acquired on a 3-Tesla MR scanner (Prisma, Siemens, Erlangen, Germany) using a 32-channel head coil. A coronal T2-weighted turbo spin echo sequence (echo time (TE)/repetition time (TR) = 85/7640 ms, flip angle = 120°, matrix size = 320 × 256, field of view (FOV) = 220 mm × 179 mm, number of slices = 43, slice thickness = 4 mm), a sagittal T1-weighted magnetization-prepared rapid acquisition gradient echo sequence (MPRAGE) (TE/TR = 2.94/2300 ms, flip angle = 9°, matrix size = 256 × 256, FOV = 256 mm × 240 mm, number of slices = 160, slice thickness = 1.2 mm) and a gradient-recalled multiecho 3D image (TE/TR = 10/52 ms, flip angle = 12°, matrix size = 448 × 448, FOV = 224 mm × 168 mm, Number of slices = 128, slice thickness = 1.0 mm) were acquired. These anatomical images were used to confirm a mild traumatic brain injury with no evidence of cerebral edema and were used for image registration. Approximately 20 min into the scan, one 10-min rs-fMRI gradient echo echo-planar imaging sequence (TE/TR = 30/2500 ms, flip angle = 90°, matrix size = 80 × 80, FOV = 240 mm × 240 mm, number of slices = 45, slice thickness = 3 mm) was performed while the individual was simply asked to remain still with eyes open. Finally, a spin echo DTI sequence (TE/TR = 79/7200 ms, matrix size = 98 × 98, FOV = 200 mm × 200 mm, number of slices = 64, slice thickness = 2 mm, b1 = 0, b2 = 1000 s/mm2, gradient directions = 64) was used for creating diffusion-weighted images.
2.4. MRI preprocessing
The rs-fMRI and DTI data were preprocessed and analyzed using FMRIB FSL software (version 5.0.9) (Jenkinson et al. 2012). The rs-fMRI data was screened for excessive motion (> 1 mm) and any subjects with a mean relative displacement >0.5 mm were either truncated to only include low motion data or were excluded. All remaining data was preprocessed using the following standard steps: motion correction by affine registration to the middle volume, brain extraction, 5 mm full width half maximum (FWHM) smoothing, and high-pass filtering at 0.01 Hz. Preprocessed data was de-noised further using single-dataset ICA with automatic dimension calculation. Components that were composed of voxels outside the brain, in the CSF, primarily white matter, with a high frequency profile or related to motion artifacts were regressed from the data (Griffanti et al. 2017). Cleaned data was registered to the corresponding MPRAGE structural T1-weighted image that was in turn registered to a standard MNI 2 mm brain-extracted standard template using linear registration with 12 degrees of freedom. Cleaned and registered rs-fMRI data were analyzed using temporally concatenated ICA to identify the ten RSNs by cross correlating with well-established reference template spatial maps (Nickerson et al. 2017; Smith et al. 2009). The default mode network (DMN) was split into anterior and posterior sections of the network and each correlated with the reference template. Dual regression algorithms were used to back-project these networks and produce RSNs at the single subject and session level (Beckmann et al. 2005). These individual RSN maps were normalized to create z-statistic images for each subject's session and each RSN. Statistical maps were down-sampled by a factor of 2 to 4 mm isotropic voxels to facilitate higher-level computational analyses (Groves et al. 2011).
DTI data was first corrected for head movement and eddy currents using the eddy tool in FSL, and then the data was masked using the non-diffusion weighted volume and a diffusion tensor was fit to each voxel. These preprocessed diffusion images were than analyzed using the tract-based spatial statistics tools (TBSS) (Smith et al. 2006). This involved further preprocessing where fractional anisotropy (FA) diffusion maps were eroded and end slices were removed. These eroded images were inspected visually for any artifacts. Images were then non-linearly registered to a 1 mm isotropic FA template image, which in turn was registered and transformed to 1 mm isotropic MNI space. All subjects' diffusion images were merged and a mean FA skeleton was made with a threshold of FA > 0.2. Each individual's diffusion maps are projected onto this skeletonized map and down-sampled to 2 mm isotropic voxels. This was repeated for each diffusion metric to generate several skeletonized maps for each subject's session(s).
2.5. Linked independent component analysis
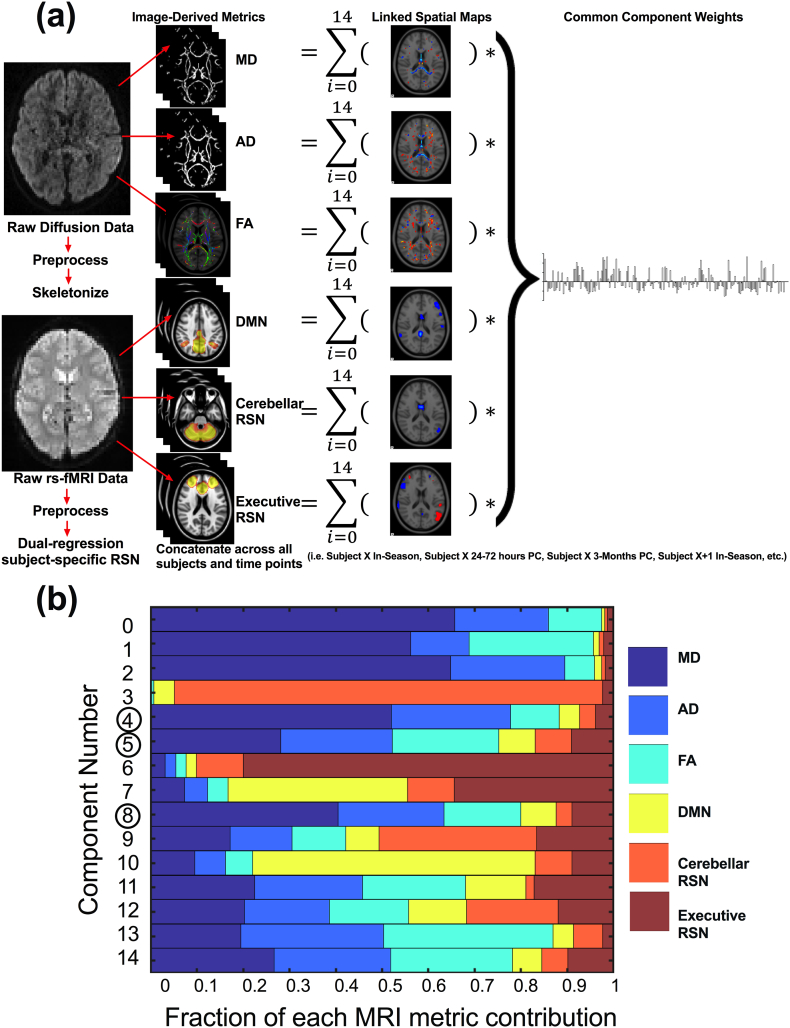
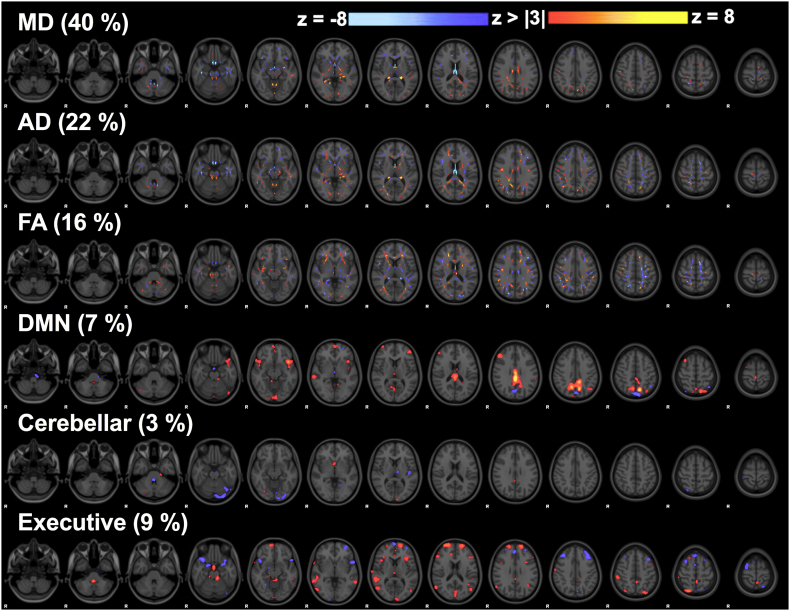
We investigated the shared inter-subject variations across 3 diffusion and 3 RSN maps to remain consistent with previous LICA applications (Groves et al. 2012; Wolfers et al. 2017). In particular, we investigated skeletonized maps of mean diffusivity (MD), axial diffusivity (AD), and fractional anisotropy (FA) maps as well as z-statistical images quantifying whole-brain voxel-wise connectivity with the posterior default mode network (DMN), cerebellar, and executive control RSNs. These networks were chosen based on previous results (Manning et al. 2017) and their potential functional relevance with respect to common concussion symptoms. An iterative LICA was performed using subject-specific spatial maps in FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLICA) with 10,000 iterations monitoring the free energy of the decomposition. A common mixing matrix containing the components' weights is common across all image-derived metrics. Data was decomposed into 15 linked independent components that are comprised of (1) spatial maps for each imaging-derived metric displaying specific regions that co-vary across subjects and (2) common component weights (Fig. 1A). Each image-derived metric of interest contributes a fractional amount to each component (Fig. 1B). The z-normalization is calculated independently for each linked independent component at every modality and the normalization factor includes the modality weights (fraction of each modality contribution) and the estimated modality-independent SNR (Groves et al. 2012). Generally n/4 is chosen as the model order, however we conservatively used a lower model order to avoid over-fitting given the longitudinal data. We repeated the analysis using ±1 model orders to confirm the robustness of our results (Wolfers et al. 2017).
Fig. 1.
Linked ICA decomposition. (A) Analysis pipeline creating MRI-derived maps of interest, and decomposing into linked components with common component weights. (B) The 15 linked components with the fraction of contribution from each MRI metric. Linked components related to concussion are circled.
2.6. Statistical analysis
Linked components were deemed clinically relevant based on significant clinical predictors of component weights or if component weights were significantly different amongst subject visits (i.e. non-concussed in-season and off-season, and concussed athletes 24–72 h, 3-months and 6-months post-concussion). We did not include components that were dominated by a single subject (i.e. describing >10% of the variance). The remaining component weights were analyzed using a linear mixed model within MATLAB (fitlme function) to determine significant predictors which included the number and severity of SCAT3 symptoms, age at the time of the scan and number of self-reported previous concussions with a fixed factor of subject visit while controlling for the longitudinal nature of the data through a random factor for subject ID number. The differences amongst subject visits was assessed using an SPSS mixed model approach with subject ID set as a random factor and age as a covariate, and post-hoc comparisons of the linearly independent estimated marginal means of component weights were assessed and corrected for multiple comparisons using Bonferroni correction. Additionally we examined repeated within-subject data over time from concussed subjects with complete data (i.e. their most recent pre-concussion scan, 24–72 h, 3-months and 6-months post-concussion). We used repeated measures one-way ANOVA with Greenhouse-Geisser corrected p-values to investigate athlete's component weights before and longitudinally post-concussion.
3. Results
3.1.1. Linked ICA decomposition
In total, 182 complete MRI datasets from 52 individual subjects were included in the LICA. We gathered 74 in-season and 63 off-season datasets and excluded six subjects for motion or data reconstruction problems (71 in- and 60 off-season) and these subjects had not experienced a concussion within 6-months (average [SD] = 0.56 [0.8] concussions ranging from 0 to 3 previous concussions that occurred on average [SD] 36 [28] months before the commencement of the study). We included 21 diagnosed concussions at 24–72 h (n = 21), then 3-month (n = 17) and 6-month (n = 13) follow-ups. Eight concussions occurred early in the season before that specific subject's in-season data could be acquired and limited repeated measures analysis. SCAT3 symptom score and severity were both significantly elevated 24–72 h post-concussion compared to both in- and off-season data and recovered by 3-months post-concussion (Table A.1, p < .0001). Three subjects experienced two diagnosed concussions over the course of the study and in all three cases full longitudinal post-concussion data relative to the first concussion could not be acquired before they experienced a second concussion. Complete longitudinal data was acquired relative to their most recent concussion. One player (#25) experienced three concussions over a three-year period and we were able to obtain three longitudinal follow-up datasets in this case, including one in-season scan before they experienced their third concussion.
A single subject drove components 3, 6, 7, and 9. Component 1 (t = −2.9, p = .003, Confidence Interval (CI) = [−0.023 to −0.044]) and 11 (t = 3.32, p = .001, CI = [0.033 to 0.13]) were significantly associated with the subject's age at the time of scanning, Table B.1). Relevant components were reproducible and correlated when replicating results for different LICA model orders (Table B.2). There were three relevant components that showed linked structure-function changes related to concussion: components 4 and 5 had a significant main effect for subject visit, components 5 was also significantly associated with the number of previous concussions (t = 2.65, p = .008, CI = [0.035 to 0.24]) and component 8 was significantly associated with SCAT3 reported symptoms (t = −2.28, p = .024, CI = [−0.07 to −0.005]).
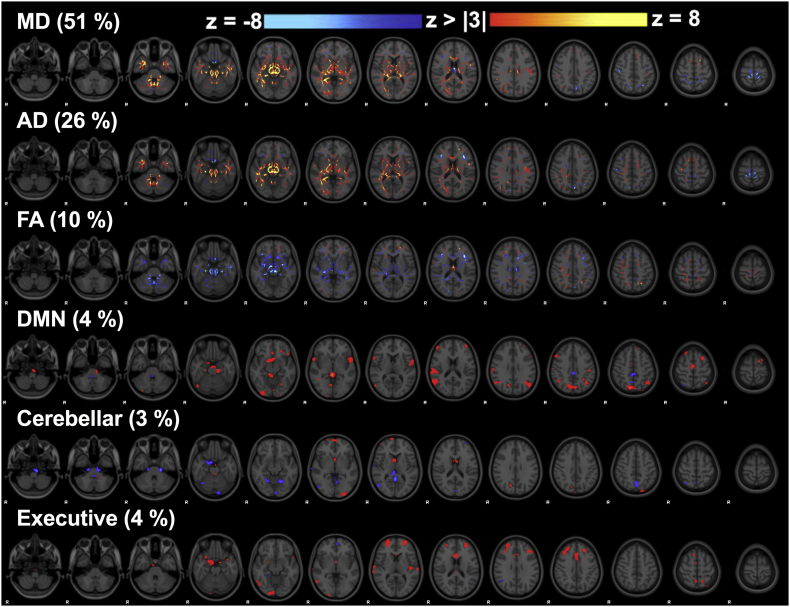
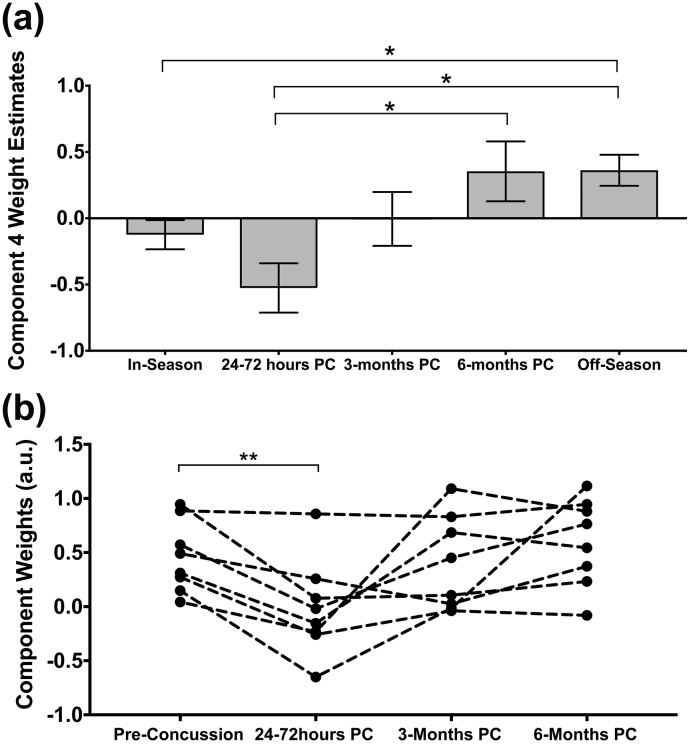
3.1.2. Linked component 4: the brain's acute response to concussion
Component 4 weights were significantly different amongst the subject visits (F = 8.13, p = .000007) and diffusion metrics dominated (87%, Fig. 1B and Fig. 2). Post-hoc tests revealed a significant difference between off-season and 24–72 h post-concussion (p = .0002) as well as between in- and off-season (p = .0009). 6-month post-concussion data was also significantly different from acute post-concussion data (p = .005). In-season and acute post-concussion data had significantly lower component weights than off-season (and 6-month data compared to acute post-concussion data only) (Fig. 3) that represent decreased MD, AD, and increased FA acutely post-concussion and during the in-season (specific regions shown in Fig. 2) that were associated with subtle changes in RSN functional connectivity. MD and AD maps overlapped with decreases acutely post-concussion focused inferiorly along the brainstem including bilateral corpus callosum, anterior thalamic radiation, inferior longitudinal and fronto-occipital fasciculus, and corticospinal tract, including the superior cerebellar peduncle (Fig. 2). FA increased in some of the same areas as MD and AD decreases. Subject #17 who notably experienced a loss of consciousness and #20 who had a relatively early previous concussion explained nearly 10% of the variance and once these two datasets were removed, the main effect (and post-hoc comparisons) remained significant. An artifact within these datasets was not evident, and their large associations with this component may be biologically relevant, i.e. related to the specific injury severity or concussion history. These datasets were not included in the repeated measures analysis for component 4.
Fig. 2.
Linked component 4. Skeletonized z-statistic maps of mean diffusivity (MD) that dominated this component, axial diffusivity (AD), and fractional anisotropy (FA) with minor contributions from the three resting state network connectivity maps.
Fig. 3.
Component 4 analysis. (A) Component weight estimated marginal means during the in- and off-season and longitudinally post-concussion (PC) with error bars depicting the standard error and (B) repeated within-subject measures ANOVA relative to their own most recent non-concussed data (dashed lines connect a single athlete's longitudinal data). Significant differences are shown with star symbol after correction for multiple comparisons.
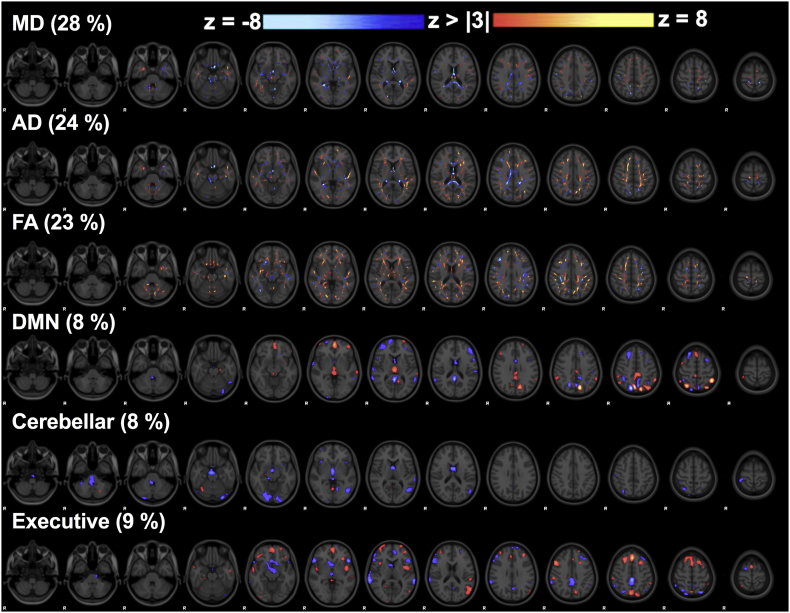
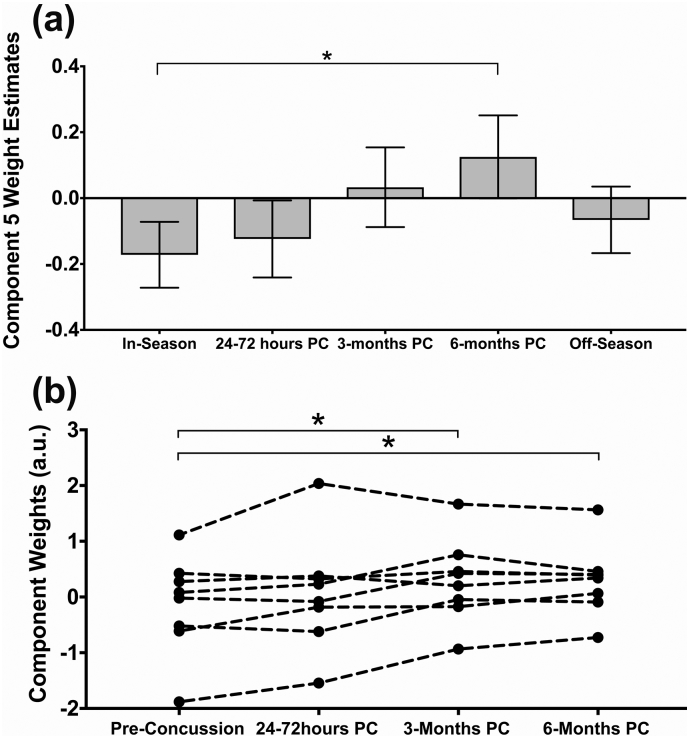
3.1.3. Linked component 5: long-term alterations of brain structure-function
Component 5 was also significantly different amongst the subject visits (F = 4.45, p = .002) and was composed of more balanced contributions from all imaging metrics (Fig. 4). There were significant differences in component 5 weight estimates between in-season and 6-months post-concussion (p = .015). Increased component 5 weights represent decreased MD and AD along the body and splenium of the corpus callosum, fornix, and thalamus linked with rs-fMRI connectivity changes (regions shown in Fig. 4). Subject #25 (concussed three times) showed a large component weight across all her time points (including in-season), and once this subject's data was removed the main effect and post-hoc result remained significant. Artifacts were once again not evident in the data and this subject's component weights may be related to her repeated concussions. This subject's data was not included in the repeated measures analysis of component 5.
Fig. 4.
Linked component 5. Z-statistic maps of co-varying mean diffusivity (MD), axial diffusivity (AD), fractional anisotropy (FA), and connectivity maps of three resting networks (DMN = default mode network) that contributed to this linked component.
3.1.4. Monitoring linked components longitudinally within individual concussed athletes
We looked at the modulation of these linked components longitudinally in concussed players with complete data (Table C.1, n = 9). There was a significant repeated measures effect (p < .05) that replicated our whole data average comparisons (Fig. 3b and 5b). We could reliably see significant decreases in component 4 weights acutely post-concussion in individual athletes compared to their specific in- or off-season scan. Component 5 weights were consistently increased at 3-months and/or 6-months post-concussion compared to their own most recent pre-concussion scan.
Fig. 5.
Component 5 analysis. (A) Component weight estimated marginal means during the in- and off-season and longitudinally post-concussion (PC) with error bars showing the standard error and (B) longitudinal within-subject component weights relative to their own most recent pre-concussion data compared using repeated measures ANOVA. Significant differences are indicated with a star symbol after correction for multiple comparisons.
3.1.5. Effects of clinical data and concussion history
Component 5 weights were significantly associated with the total number of concussion reported (t = 2.65, p = .009, CI = [0.035 to 0.236]). The number of reported SCAT3 symptoms was significantly associated with component 8 weights (t = −2.38, p = .018, CI = [−0.07 to −0.005]) within the linear mixed effects model. MD dominated this linked component once again focused in the fornix, corpus callosum and thalamus, with co-varying AD, FA, DMN and executive RSN connectivity changes (Fig. 6).
Fig. 6.
Linked component 8. The number of SCAT3 symptoms reported was significantly associated with component 8 weights using a linear mixed effects model.
4. Discussion
In this study, we used data-driven techniques to examine female rugby players with and without a concussion over time. This is the first concussion study to examine multiparametric MRI data using LICA and allowed unbiased and reliable decomposition of the data to reveal complex neurobiological signatures that characterized structure-function alterations both acutely and persistently post-concussion. These linked changes of brain structure-function were related to self-reported concussion history and consistent when following individual concussed athletes longitudinally over time.
Rugby generally does not involve direct impacts to the head but rather substantial and repetitive rotational accelerations of the head. A study of amateur male rugby players reported 77 impacts exceeding 10 g acceleration per game, and higher rotational accelerations compared to other previously studied cohorts like football (King et al. 2015). A recent study of female rugby players recorded an average of 14 impacts per player, per match and the majority of these were to the side of the head (King et al. 2018). We expect that these numbers are not substantially different for the varsity athletes studied here given the rigorous practice schedule (4 contact practices/week) where they may experience subconcussive (and concussive) impacts and accelerations.
We found two linked multiparametric MRI components that were expressed differently amongst the subject visits. Component 4 was associated with acute brain changes post-concussion and during the in-season where athletes participate in contact practices and play. Diffusion metrics dominated this component and corresponded to decreased MD and AD located inferiorly in the brain involving many deep white matter tracts linked with rs-fMRI connectivity decreases between cortical regions in the frontal, temporal and occipital lobes. This included regions structurally connected by the implicated white matter tracts as well as regions spatially near diffusion variations.
Acute decreases in MD and AD have been observed post-concussion in multiple studies, and could reflect neuroinflammation, diffuse axonal disruption, and/or changes in myelin content (Bazarian et al. 2007; Chu et al. 2010; Mayer et al. 2012; Wilde et al. 2008). We observed that this linked component recovered back to off-season levels by 3-months post-concussion and was also not strongly associated with reorganized functional connectivity patterns. This component may reflect an acute neuroinflammatory response and/or a temporary disruption of inferiorly located white matter tracts near the brainstem. In our previous study of younger hockey players, we also found changes in white matter diffusion located inferiorly in the corticospinal tract within 24–72 h post-concussion with very subtle changes in functional connectivity (Manning et al. 2017) as well as acute neuroinflammatory blood biomarkers (Daley et al. 2016).
In-season data had decreased diffusion compared to off-season data (the same direction as the acute post-concussion data) and could reflect the cumulative effect of subconcussive impacts (Abbas et al. 2015; Slobounov et al. 2017). Previous studies support this interpretation by relating accelerometer measures of repetitive head impacts with diffusion MRI measures in non-concussed contact sport athletes (McAllister et al. 2014). MR spectroscopy data in a subset of these rugby players has been published previously and reported glutamine decreases within the prefrontal white matter of both non-concussed and concussed rugby players (Schranz et al. 2017). Given the present findings, we are acquiring data from age-matched varsity female rowers and swimmers (non-contact) in order to control for the influence of high athletic activity.
Component 5 represented linked alterations in brain microstructure and functional connectivity that persisted beyond symptomatic recovery post-concussion. Decreases in diffusion along major white matter tracts like the corpus callosum and co-varying changes in rs-fMRI network connectivity were observed at 6-months post-concussion. Some of these changes are consistent with previous longitudinal findings of a small study of concussed female athletes (Chamard et al. 2016). A decrease in AD has been shown to correlate with damage to the axons themselves due to rotationally accelerated stretch-injury and the corpus callosum has been repeatedly shown to be vulnerable to concussion (Chamard et al. 2016; Stamm et al. 2015; Sullivan et al. 2013; Wright et al. 2016). The fornix is another major white matter structure that carries information from the hippocampus and limbic system in general, and has been associated with memory loss and CTE (Adnan et al. 2013; McKee et al. 2013; Thomas et al. 2011). These linked longitudinal imaging changes represent simultaneous disruption of white matter tracts and compensatory functional connectivity changes that may reflect simultaneous membrane dysfunction and physical alterations of microstructure that consequently affect functional architecture. The number of concussions reported was also significantly associated with the component weights. There may be inaccuracies in this self-reported data and undiagnosed concussions that the players did not seek medical attention for. However, given recent research findings of severe CTE in young varsity athletes (Mez et al. 2017), this is an important result that requires further investigation. Our data suggests that concussion causes long-lasting and cumulative changes of brain microstructure and functional connectivity at 6-months or even years after injury. Abnormalities within central and primary white matter structures appear to be closely related to concussion history and are linked with altered functional connectivity.
Using conjunction analysis approaches, we can appreciate that these long-term post-concussion disruptions along fundamental white matter tracts are directly linked with alterations in functional connectivity throughout the cortex. Component 8 was significantly related to the SCAT3 symptom score and involved diffusion measures in the fornix linked with DMN and executive RSN hyperconnectivity. Functional hyperconnectivity patterns were not exclusively evident at 3-months post-concussion like in our previous study of younger male hockey players (Manning et al. 2017), however prolonged decreases in MD and AD were observed in both studies. Comparison between these two studies is difficult due to many different factors including age, sex, analytical approach, the use of a helmet, or sports-specific biomechanical factors. Future research efforts should investigate if these brain alterations are a precursor of neurodegenerative processes or if they can enable more specific predictions of recovery and vulnerability to re-injury. While this data-driven approach has a number of advantages, there are a few subjective choices including the chosen model order and what parameters to input into the model that are a limitation, where other choices may render different results. In general the DTI metrics dominated the linked components that were significantly altered longitudinally post-concussion. We also found that components that were dominated by a single subject's data were often a result of a specific RSN connectivity map, perhaps indicating that the DTI metrics are spatially more consistently altered post-concussion compared to the resting state connectivity patterns explored here. Another limitation is the relatively small sample size of concussed athletes and these findings warrant further investigation and replication.
The conjunction of MRI data from this complex cohort of female rugby players has shown that there are acute multiparametric imaging changes following concussion. While some of these changes may reflect acute neuroinflammatory disruptions that recover quickly along with symptoms, we found linked functional and white matter microstructural changes that remained altered 6-months after the concussion - well beyond clinical recovery and clearance to return to play. Linked imaging components related to individual concussion history where currently healthy subjects with more previous concussions were related to decreases in AD within the fornix and corpus callosum linked with both structurally connected and distal cortical connectivity changes. Though some components related to clinical scores, the associated long-term clinical implication of these observations remains an open question. However, given the growing literature and the findings reported here, it is clear that concussions are related to underlying white matter microstructural and linked functional connectivity changes that persist well beyond symptomatic recovery.
Acknowledgments
Acknowledgements
The authors would to thank the athletes who participated in this longitudinal study and the coaching staff. We would also like to acknowledge the support provided by the MRI technicians and staff at the Centre for Functional and Metabolic Mapping. We acknowledge funding through the Schulich School of Medicine and Dentistry, Western University; Brain Canada; Canada First Research Excellence Fund; and the Natural Sciences and Engineering Research Council of Canada.
Author contributions
KYM was responsible for analysis and interpretation of the data, writing and revising the manuscript. RSM was responsible for image acquisition design and interpretation of the data. AL and CFB provided feedback and guidance on the analysis approach. CB and KB coordinated informed consent and study participant appointments. LF and TJ were responsible for patient evaluation and JH for clinical acquisition. RB, GAD, AB, LF, TJD, DDF, JH, and RSM were responsible for overall study concept and design, and all authors provided critical feedback of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.101627.
Contributor Information
Kathryn Y. Manning, Email: kmanning@robarts.ca.
Alberto Llera, Email: a.llera@donders.ru.nl.
Gregory A. Dekaban, Email: dekaban@robarts.ca.
Robert Bartha, Email: rbartha@robarts.ca.
Christy Barreira, Email: cbarreira@robarts.ca.
Arthur Brown, Email: abrown@robarts.ca.
Lisa Fischer, Email: lfischer@uwo.ca.
Tatiana Jevremovic, Email: tjevremo@uwo.ca.
Kevin Blackney, Email: kblackne@uwo.ca.
Timothy J. Doherty, Email: tim.doherty@lhsc.on.ca.
Douglas D. Fraser, Email: douglas.fraser@lhsc.on.ca.
Jeff Holmes, Email: jholme@uwo.ca.
Christian F. Beckmann, Email: c.beckmann@donders.ru.nl.
Ravi S. Menon, Email: rmenon@robarts.ca.
Appendix A. Supplementary data
Supplementary material
References
- Abbas K., Shenk T.E., Poole V.N., Breedlove E.L., Leverenz L.J., Nauman E.A., Talavage T.M., Robinson M.E. Alteration of default mode network in high school football athletes due to repetitive subconcussive mild traumatic brain injury: a resting-state functional magnetic resonance imaging study. Brain Connect. 2015;5:91–101. doi: 10.1089/brain.2014.0279. [DOI] [PubMed] [Google Scholar]
- Adnan A., Crawley A., Mikulis D., Moscovitch M., Colella B., Green R. Moderate–severe traumatic brain injury causes delayed loss of white matter integrity: evidence of fornix deterioration in the chronic stage of injury. Brain Inj. 2013;27:1415–1422. doi: 10.3109/02699052.2013.823659. [DOI] [PubMed] [Google Scholar]
- Alosco M.L., Kasimis A.B., Stamm J.M., Chua A.S., Baugh C.M., Daneshvar D.H., Robbins C.A., Mariani M., Hayden J., Conneely S., Au R., Torres A., McClean M.D., McKee A.C., Cantu R.C., Mez J., Nowinski C.J., Martin B.M., Chaisson C.E., Tripodis Y., Stern R.A. Age of first exposure to American football and long-term neuropsychiatric and cognitive outcomes. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian J.J., Zhong J., Blyth B., Zhu T., Kavcic V., Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Deluca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. B Biol. Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler E.D., Maxwell W.L. Neuropathology of mild traumatic brain injury: Relationship to neuroimaging findings. Brain Imaging Behav. 2012;6:108–136. doi: 10.1007/s11682-011-9145-0. [DOI] [PubMed] [Google Scholar]
- Chamard E., Lefebvre G., Lassonde M., Theoret H. Long-Term abnormalities in the corpus callosum of female concussed athletes. J. Neurotrauma. 2016;33:1220–1226. doi: 10.1089/neu.2015.3948. [DOI] [PubMed] [Google Scholar]
- Chu Z., Wilde E.A., Hunter J.V., McCauley S.R., Bigler E.D., Troyanskaya M., Yallampalli R., Chia J.M., Levin H.S. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. Am. J. Neuroradiol. 2010;31:340–346. doi: 10.3174/ajnr.A1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill N., Hutchison M., Richards D., Leung G., Graham S., Schweizer T.A. Brain structure and function associated with a history of sport concussion: a multi-modal magnetic resonance imaging study. J. Neurotrauma. 2017;34:765–771. doi: 10.1089/neu.2016.4531. [DOI] [PubMed] [Google Scholar]
- Daley M., Dekaban G., Bartha R., Brown A., Stewart T.C., Doherty T., Fischer L., Holmes J., Menon R.S., Rupar C.A., Shoemaker J.K., Fraser D.D. Metabolomics profiling of concussion in adolescent male hockey players: a novel diagnostic method. Metabolomics. 2016;12(185) [Google Scholar]
- Griffanti L., Douaud G., Bijsterbosch J., Evangelisti S., Alfaro-Almagro F., Glasser M.F., Duff E.P., Fitzgibbon S., Westphal R., Carone D., Beckmann C.F., Smith S.M. Hand classification of fMRI ICA noise components. NeuroImage. 2017;154:188–205. doi: 10.1016/j.neuroimage.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A.R., Beckmann C.F., Smith S.M., Woolrich M.W. Linked independent component analysis for multimodal data fusion. NeuroImage. 2011;54:2198–2217. doi: 10.1016/j.neuroimage.2010.09.073. [DOI] [PubMed] [Google Scholar]
- Groves A.R., Smith S.M., Fjell A.M., Tamnes C.K., Walhovd K.B., Douaud G., Woolrich M.W., Westlye L.T. Benefits of multi-modal fusion analysis on a large-scale dataset: Life-span patterns of inter-subject variability in cortical morphometry and white matter microstructure. NeuroImage. 2012;63:365–380. doi: 10.1016/j.neuroimage.2012.06.038. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kamins J., Bigler E., Covassin T., Henry L., Kemp S., Leddy J.J., Mayer A., McCrea M., Prins M., Schneider K.J., Valovich McLeod T.C., Zemek R., Giza C.C. What is the physiological time to recovery after concussion? A systematic review. Br. J. Sports Med. 2017;51:935–940. doi: 10.1136/bjsports-2016-097464. [DOI] [PubMed] [Google Scholar]
- King D., Hume P.A., Brughelli M., Gissane C. Instrumented mouthguard acceleration analyses for head impacts in amateur rugby union players over a season of matches. Am. J. Sports Med. 2015;43:614–624. doi: 10.1177/0363546514560876. [DOI] [PubMed] [Google Scholar]
- King D.A., Hume P.A., Gissane C., Kieser D.C., Clark T.N. Head impact exposure from match participation in women's rugby league over one season of domestic competition. J. Sci. Med. Sport. 2018;21:139–146. doi: 10.1016/j.jsams.2017.10.026. [DOI] [PubMed] [Google Scholar]
- Manning K.Y., Schranz A., Bartha R., Dekaban G.A., Barreira C., Brown A., Fischer L., Asem K., Doherty T.J., Fraser D.D., Holmes J., Menon R.S. Multiparametric MRI changes persist beyond recovery in concussed adolescent hockey players. Neurology. 2017;89:2157–2166. doi: 10.1212/WNL.0000000000004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R., Ling J.M., Yang Z., Pena A., Yeo R.A., Klimaj S. Diffusion abnormalities in pediatric mild traumatic brain injury. J. Neurosci. 2012;32:17961–17969. doi: 10.1523/JNEUROSCI.3379-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T.W., Ford J.C., Flashman L.A., Maerlender A., Greenwald R.M., Beckwith J.G., Bolander R.P., Tosteson T.D., Turco J.H., Raman R., Jain S. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology. 2014;82:63–69. doi: 10.1212/01.wnl.0000438220.16190.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A.C., Stein T.D., Nowinski C.J., Stern R.A., Daneshvar D.H., Alvarez V.E., Lee H.S., Hall G., Wojtowicz S.M., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W., Cantu R.C. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mez J., Daneshvar D.H., Kiernan P.T., Abdolmohammadi B., Alvarez V.E., Huber B.R., Alosco M.L., Solomon T.M., Nowinski C.J., McHale L., Cormier K.A., Kubilus C.A., Martin B.M., Murphy L., Baugh C.M., Montenigro P.H., Chaisson C.E., Tripodis Y., Kowall N.W., Weuve J., McClean M.D., Cantu R.C., Goldstein L.E., Katz D.I., Stern R.A., Stein T.D., McKee A.C. Clinicopathological evaluation of chronic traumatic encephalopathy in players of american football. JAMA. 2017;318:360–370. doi: 10.1001/jama.2017.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson L.D., Smith S.M., Öngür D., Beckmann C.F. Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Front. Neurosci. 2017;11(115) doi: 10.3389/fnins.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCAT3 Br. J. Sports Med. 2013;47:259. [PubMed] [Google Scholar]
- Schranz A., Manning K.Y., Dekaban G.A., Fischer L., Jevremovic T., Blackney K., Barreira C., Doherty T., Fraser D., Brown A., Holmes J., Menon R.S., Bartha R. Reduced brain glutamine in female varsity rugby athletes after concussion and in non-concussed athletes after a season of play. Hum. Brain Mapp. 2017 doi: 10.1002/hbm.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S.S., Bales J.W., Edward Dixon C., Hwang M. Structural imaging of mild traumatic brain injury may not be enough: overview of functional and metabolic imaging of mild traumatic brain injury. Brain Imaging Behav. 2017:1–20. doi: 10.1007/s11682-017-9684-0. [DOI] [PubMed] [Google Scholar]
- Slobounov S.M., Walter A., Breiter H.C., Zhu D.C., Bai X., Bream T., Seidenberg P., Mao X., Johnson B., Talavage T.M. The effect of repetitive subconcussive collisions on brain integrity in collegiate football players over a single football season: a multi-modal neuroimaging study. NeuroImage Clin. 2017;14:708–718. doi: 10.1016/j.nicl.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., MacKay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E.J. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., MacKay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm J.M., Koerte I.K., Muehlmann M., Pasternak O., Bourlas A.P., Baugh C.M., Giwerc M.Y., Zhu A., Coleman M.J., Bouix S., Fritts N.G., Martin B.M., Chaisson C., McClean M.D., Lin A.P., Cantu R.C., Tripodis Y., Stern R.A., Shenton M.E. Age at first exposure to football is associated with altered corpus callosum white matter microstructure in former professional football players. J. Neurotrauma. 2015;32:1768–1776. doi: 10.1089/neu.2014.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Adali T., Yu Q., Chen J., Calhoun V.D. A review of multivariate methods for multimodal fusion of brain imaging data. J. Neurosci. Methods. 2012;204:68–81. doi: 10.1016/j.jneumeth.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G.M., Mierzwa A.J., Kijpaisalratana N., Tang H., Wang Y., Song S.-K., Selwyn R., Armstrong R.C. Oligodendrocyte lineage and subventricular zone response to traumatic axonal injury in the corpus callosum. J. Neuropathol. Exp. Neurol. 2013;72:1106–1125. doi: 10.1097/NEN.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.G., Koumellis P., Dineen R.A. The fornix in health and disease: an imaging review. Radiographics. 2011;31:1107–1121. doi: 10.1148/rg.314105729. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., McCauley S.R., Hunter J.V., Bigler E.D., Chu Z., Wang Z.J., Hanten G.R., Troyanskaya M., Yallampalli R., Li X., Chia J., Levin H.S. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- Wolfers T., Arenas A.L., Onnink A.M.H., Dammers J., Hoogman M., Zwiers M.P., Buitelaar J.K., Franke B., Marquand A.F., Beckmann C.F. Refinement by integration: aggregated effects of multimodal imaging markers on adult ADHD. J. Psychiatry Neurosci. 2017;42:386–394. doi: 10.1503/jpn.160240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A.D., Jarrett M., Vavasour I., Shahinfard E., Kolind S., van Donkelaar P., Taunton J., Li D., Rauscher A. Myelin water fraction is transiently reduced after a single mild traumatic brain injury – a prospective Cohort study in collegiate hockey players. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material