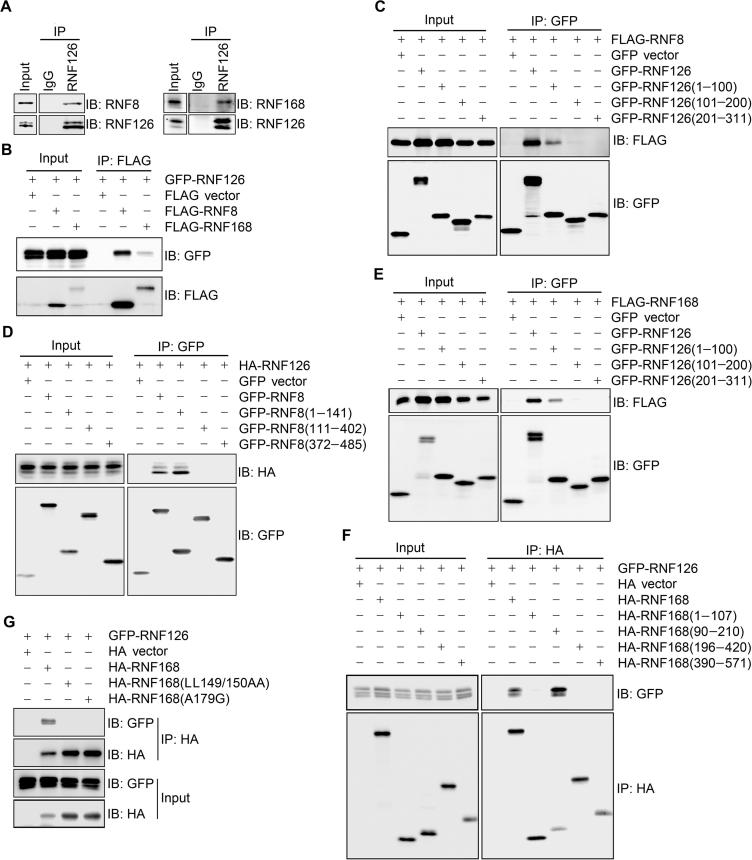
Figure 2.
RNF126 interacts with RNF8 and RNF168
A. Endogenous RNF126 interacts with both RNF8 and RNF168. Total lysates from HEK293T cells were immunoprecipitated with an anti-RNF126 antibody, and the immunocomplexes were exposed to the indicated antibodies. B. Epitope-tagged RNF126 interacts with RNF8 and RNF168. A GFP-RNF126 expression construct was co-transfected with FLAG-RNF8 or FLAG-RNF168 in HEK293T cells. Total lysates were harvested 48 h after transfection and subjected to immunoprecipitation with anti-FLAG beads followed by immunoblotting with the indicated antibodies. C. The RNF126 N-terminus (amino acid residues 1–100) interacts with RNF8. FLAG-RNF8 was co-expressed with GFP-RNF126 or its truncation mutants in HEK293T cells. D. The FHA domain-containing N-terminus of RNF8 (amino acid residues 1–141) interacts with RNF126. HA-RNF126 was co-expressed with GFP-RNF8 or its truncation mutants in 293T cells. E. The RNF126 N-terminus interacts with RNF168. FLAG-RNF168 was co-expressed with GFP-RNF126 or its truncation mutants in HEK293T cells. F. The UMI and MIU1 domains-containing region of RNF168 (amino acid residues 90–210) interact with RNF126. GFP-RNF126 was co-expressed with HA-RNF168 or its truncation mutants in HEK293T cells. G. Both UMI domain and MIU1 domain of RNF168 are essential for its interaction with RNF126. GFP-RNF126 was co-expressed with wild-type HA-RNF168, the UMI point mutant HA-RNF168(LL149/150AA), or the MIU1 point mutant HA-RNF168(A179G) in HEK293T cells. In panels C–G, total cell lysates were harvested 48 h after transfection and subjected to immunoprecipitation and immunoblotting with the indicated antibodies. IB, immunoblot; IP, immunoprecipitation; FHA, forkhead-associated; MIU1, motif interacting with ubiquitin 1; UMI, ubiquitin interacting motif and MIU-related ubiquitin binding domain.