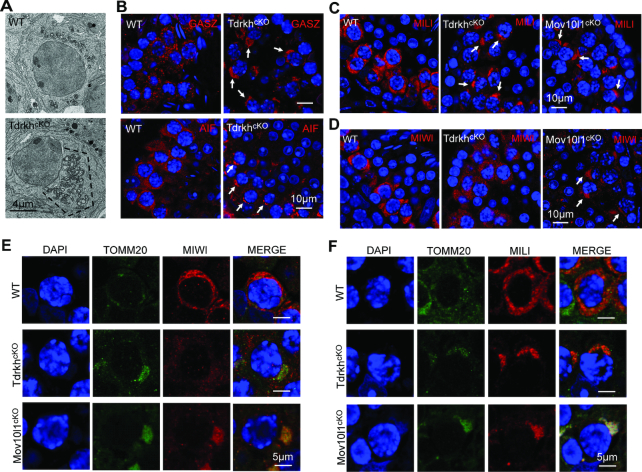
Figure 4.
TDRKH deficiency selectively disrupts the localization of MIWI to mitochondria in spermatocytes. (A) Loss of TDRKH causes the aggregation of mitochondria in spermatocytes. Transmission electron microscopy was performed on pachytene spermatocytes from adult WT and TdrkhcKO testes. The mitochondria aggregation is indicated by dotted line. Scale bar, 4 μm. (B) TDRKH deficiency causes the aggregation of mitochondrial proteins GASZ and AIF in spermatocytes. Immunostaining was performed using indicated antibodies on adult WT and TdrkhcKO testes. DNA was stained with DAPI. Protein aggregations are indicated by arrows. Scale bar, 10 μm. (C, D) TDRKH deficiency causes the aggregation of MILI (C), but not MIWI (D), in spermatocytes. Immunostaining was performed using indicated antibodies on adult WT, TdrkhcKO and Mov10l1cKO testes. DNA was stained with DAPI. Protein aggregations are indicated by arrows. Scale bar, 10 μm. (E) Loss of TDRKH disrupts the localization of MIWI to mitochondria. Co-immunostaining was performed using MIWI and TOMM20 antibodies on adult WT, TdrkhcKO and Mov10l1cKO testes. DNA was stained with DAPI. TOMM20 served as a mitochondrial marker. Scale bar, 5 μm. (F) The congregation of MILI in TdrkhcKOand Mov10l1cKOtestes. Co-immunostaining was performed using MILI and TOMM20 antibodies on adult WT, TdrkhcKO and Mov10l1cKO testes. DNA was stained with DAPI. TOMM20 served as a mitochondrial marker. Scale bar, 5 μm.