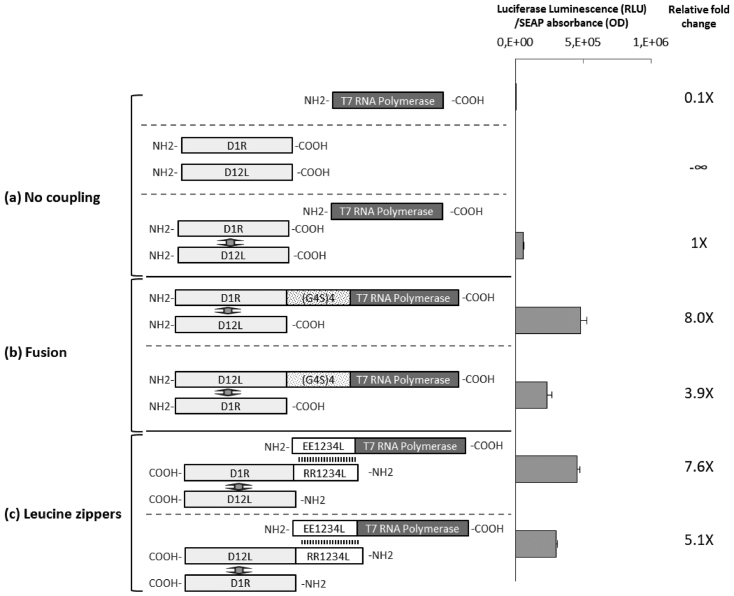
Figure 2.
Coupling of T7RNAP with vaccinia virus capping enzyme. Plasmids encoding T7RNAP and/or the D1R and D12L subunits of the heterodimeric vaccinia virus capping enzyme were cotransfected in HEK-293 cells together with the pT7φ10-Luciferase plasmid. The ORFs of corresponding plasmids were designed as follows: (A) no coupling domain, (B) T7RNAP fused in-frame to the carboxyl-terminal end of either D1R or D12L genes via the (G4S)4 linker, (C) fused in-frame with the leucine-zippers EE1234L and RR1234L, which forms a heterodimer in antiparallel orientation. EE1234L was fused to the amino-terminal end of T7RNAP, whereas RR1234L was fused either with D1R or D12L. Luciferase gene expression level is shown, as well as the relative value in comparison to the uncoupled condition. Luminescence was assayed in cell lysates 24 h after transfection and expressed in RLU normalized for hSEAP (secreted embryonic alkaline phosphatase) expression expressed in DO (RLU/DO ratio). Errors bars indicate standard deviation of at least four experiments. Comparisons of cotransfection of T7RNAP, D1R and D12L versus all other constructions: P< 0.05, Student's t-test.